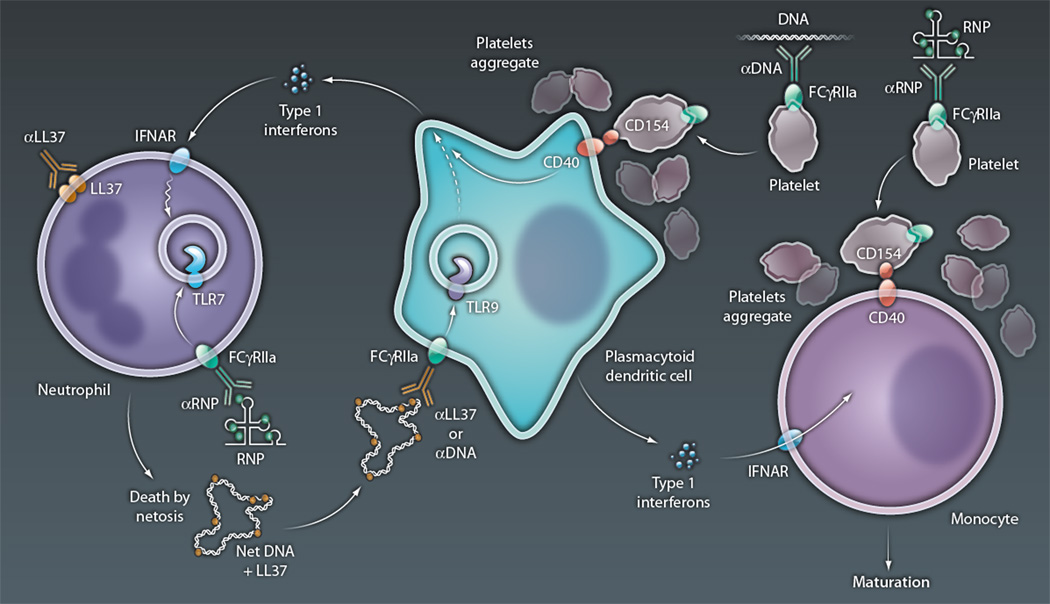
Figure 1. Immune cell medley in SLE.
Type I interferons, released from pDCs in SLE, prime neutrophils for death by NETosis, with up-regulation of TLR7 in endosomes and translocation of microbial peptides (such as LL37) to the cell surface. NETosis is then initiated by FcR-mediated uptake of anti-RNP and RNP immune complexes, with the RNA component of the latter engaging TLR7 in conjunction with release of ROS required for NET formation, and by the binding of surface-associated LL37 by anti-LL37 autoantibodies, presumably also leading to ROS release. NETs released from dying neutrophils are then taken up by pDCs, directly or as an immune complex of NET-associated LL37 and DNA, along with anti-LL37 or anti-DNA autoantibodies. In either case, the DNA component of the NETs engages TLR9 in endosomes, with the activation of genes that lead to interferon release and thus further neutrophil priming. Immune complexes of anti-DNA and DNA, or anti-RNP and RNPs, also activate platelets via an FcR-mediated pathway, with up-regulation of surface CD154 and aggregation. Aggregated platelets form complexes with pDCs and monocytes, enabling CD40-mediated activation after its engagement by platelet-expressed CD154. CD40 activation of pDCs leads to interferon release, whereas that of monocytes promotes their maturation to antigen-presenting dendritic cells, a process also enabled by interferons released by pDCs. The latter APCs serve as efficient activators of autoreactive T cells in lupus, which can promote release of autoantibodies (anti-LL37, anti-RNPs, and anti-DNA) by autoreactive B cells (not shown).
CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE