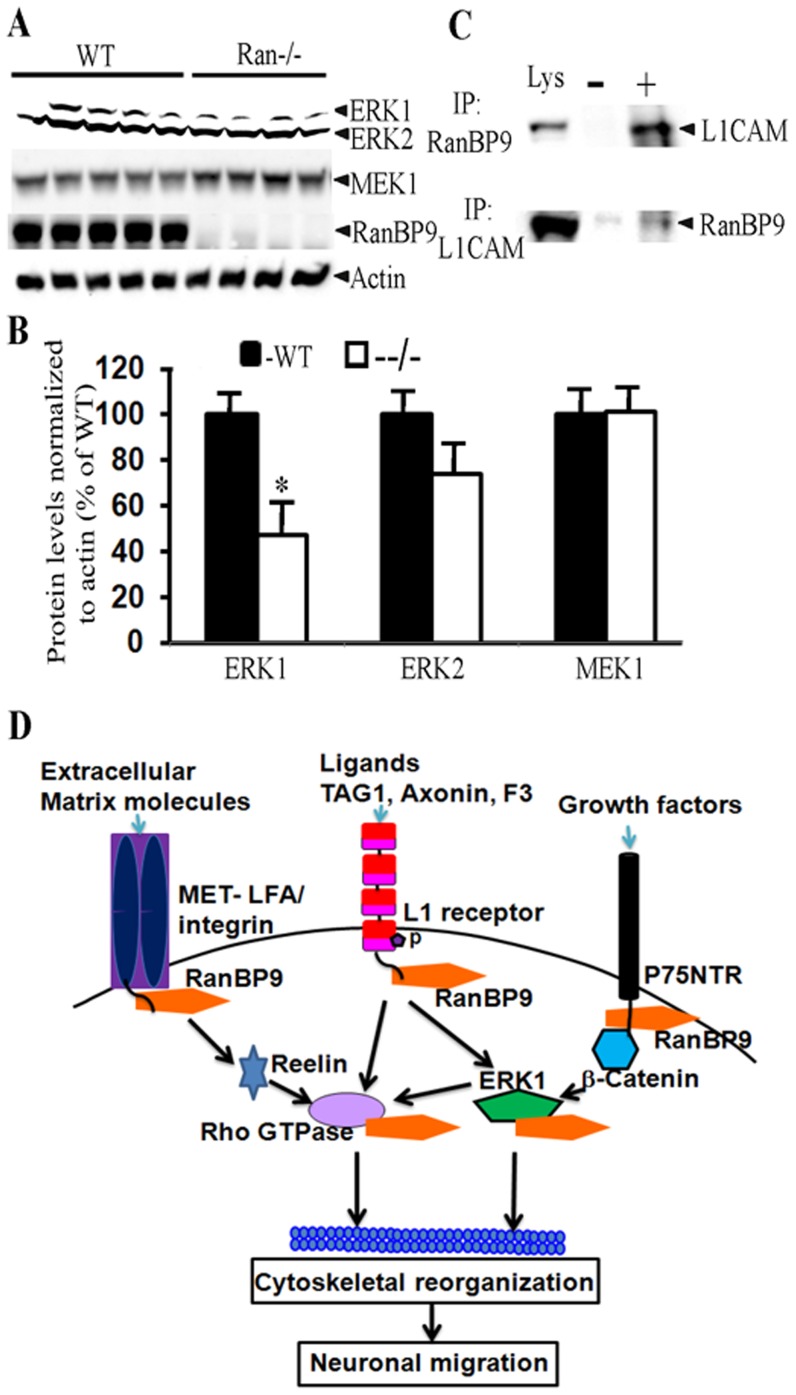
Figure 10. ERK1 protein levels are significantly reduced in Ran−/− brains from P1 mice.
(A), Brain lysates from WT and Ran−/−mice were subjected to SDS-PAGE electrophoresis and ERK1/ERK2 & MEK1 proteins were detected using their specific polyclonal antibodies. Actin blot is shown as loading control and RanBP9 blot shows complete absence of RanBP9 protein in Ran−/− pups. (B) Quantitation of signal intensities by imageJ revealed significantly reduced protein levels for ERK1 (47%). n = 5 (WT) and 4 (Ran−/−),±SEM. *, p<0.05 by t test. (C), Endogenous L1CAM was pulled down by RanBP9 antibody (upper panel) and in a reciprocal coimmunopreciptation experiments, RanBP9 was pulled down by L1CAM antibodies (lower panel) suggesting that both L1CAM and RanBP9 interact with each other. (D), A model to explain mechanism of brain growth defects in Ran−/− brains. In response to cell adhesion signals, RanBP9 scaffolds L1CAM and ERK1 thereby activating downstream effectors such as Rho GTPases. Rho GTPases are known to physically interact and modulate cytoskeletal elements, which in turn alter neuronal differentiation/migration. RanBP9 is also known to bind MET-LFA integrin and p75NTR suggesting that alternative pathways to modulate differentiation/migration are also possible.