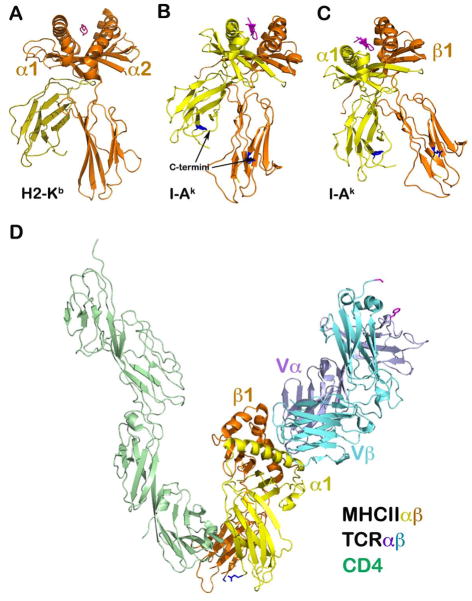
Fig. 12. Overall topology of the CD4/TCR/pMHCII ternary complex.
(A) A ‘classic’ view of the MHCI molecule demonstrating how an MHC molecule on an APC presents the antigenic peptide vertically toward an opposing T cell for TCR recognition. The light chain, β2 microglobulin (yellow) is non-covalently attached to the heavy chain. (B) A similar ‘classic’ view of MHCII molecule as that of MHCI in (A). In this view, the α subunit in yellow has its C-terminal α2 domain residue (dark blue stick model) high above the plasma membrane relative to the C-terminal residue (dark blue stick model) of the β2 domain in orange. (C) Revised MHCII orientation. Given that the stalks of both the α and β subunits are ~10-residue long, the stalk and TM should impose constraints on the two subunits such that the MHCII molecule must be oriented in a manner more tilted compared to the view shown in (B). As a consequence, the two MHC terminal residues are at about the same height on the membrane. The helical region of the β1 domain would significantly project upward in comparison to that of the α1 domain. (D) The overall orientation of the ternary complex (coordinates from PDB 3T0E). This representation takes into account the V-shaped CD4-MHCII architecture as well as the MHCII topology on the APC membrane given in (C) above. It becomes obvious how the MHC class II-restricted TCR must contact the MHCII molecule tangentially from the right in the figure. The TCRαβ will ‘bump’ up against the ‘wall’ or barrier of the helical region of the β1 domain to provide a mechanical force, supporting T-cell mechanotransduction. Note that although the CPs are not shown from the T-cell side, CD4 has a shorter stalk than that of the TCRαβ heterodimer subunits.