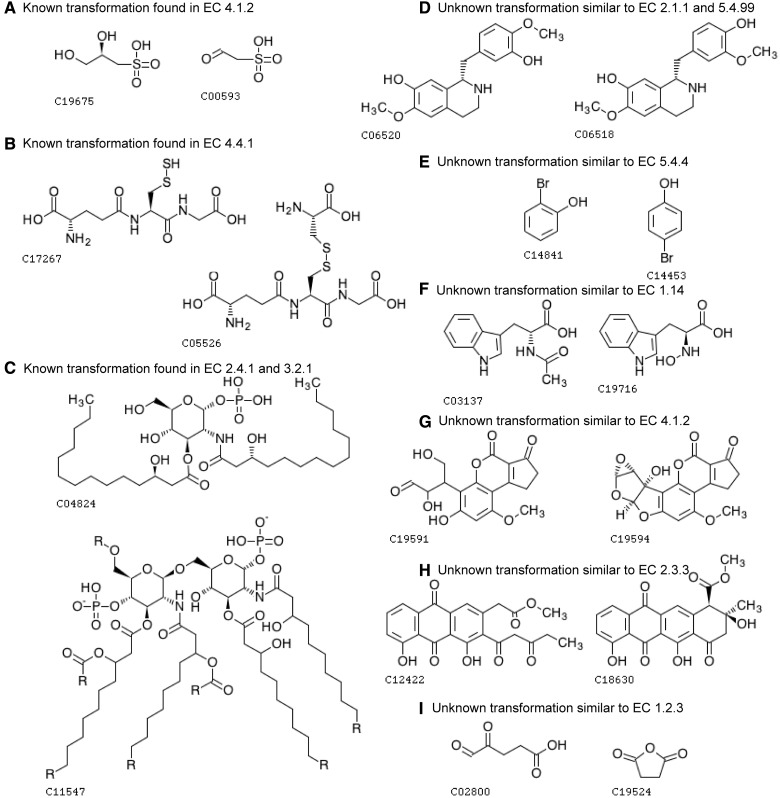
Fig. 6.
Examples of the predicted pairs taken from Figure 5. The chemical transformation patterns in pairs (A–C) are already known and described in KEGG reactant pairs (Note that these reactions are not known, but the transformation patterns are known), whereas pairs (D–I) have unknown patterns. (A) C-C bond accompanied with secondary alcohol group is degraded and forms an aldehyde group, which is a reaction typically found in EC sub-subclass 4.1.2 (aldehyde-lyases). (B) C-S bond in disulfide bond is degraded and forms an S-mercapto group, which is found in EC sub-subclass 4.4.1 (carbon-sulfur lyases). (C) This chemical transformation pattern is found in many reactions in EC 2.4.1 (glycosyltransferases) and EC 3.2.1 (glycosidases). (D) This pattern is not found in known reactions. At the first sight, this pair may look like two steps of methylation/demethylation (EC 2.1.1) or intramolecular transfer of a methyl group (part of EC 5.4). With closer investigations of Isoquinoline alkaloid biosynthesis pathway (map00950, which these compounds belong to), it looks more natural to occur the two steps of metylenedioxy ring formation/cleavage (EC 1.14.21 or 1.21.3) because some metylenedioxy ring formation reactions are known to take place in this pathway. However, in any case, methylation and metylenedioxy ring formation occurs in the context of biosynthesis, whereas demethylation and metylenedioxy ring cleavage occurs in the context of biodegradation. In that sense, this compound–compound pair may be an example of false positives when taking account of the reaction flow in the pathway level. (E) This compound–compound pair may look intramolecular transfer of a hydroxy group, which is typically found in EC 5.4.4 (hydroxymutases), but the transfer of hydroxy group from a position to another in an aromatic ring is not found in any known reactions stored in KEGG. This pair may be another example of false positives because the substitution of hydroxy group in aromatic ring is much harder to occur than the addition of hydroxy group. It is known that some anaerobic bacteria have 4-hydroxybenzoyl-CoA reductase (EC 1.3.7.9) that catalyzes the substitution of hydroxy group in aromatic ring. However, we assume it would be hard to catalyze intramolecular transfer of hydroxy group in substituted aromatic ring. (F) Although there are many varieties of hydroxylases (part of EC 1.14), there is no known pattern to produce hydroxyl amine from amide group. (G) For this reaction to occur, there need to be more than one reaction steps, and an important step would be similar to EC 4.1.2 (aldehyde-lyases). (H) There are similar EC 2.3.3 (acyl transferases) reactions in polyketide synthesis. (I) Some of EC 1.2.3 (oxidases) catalyze similar reactions