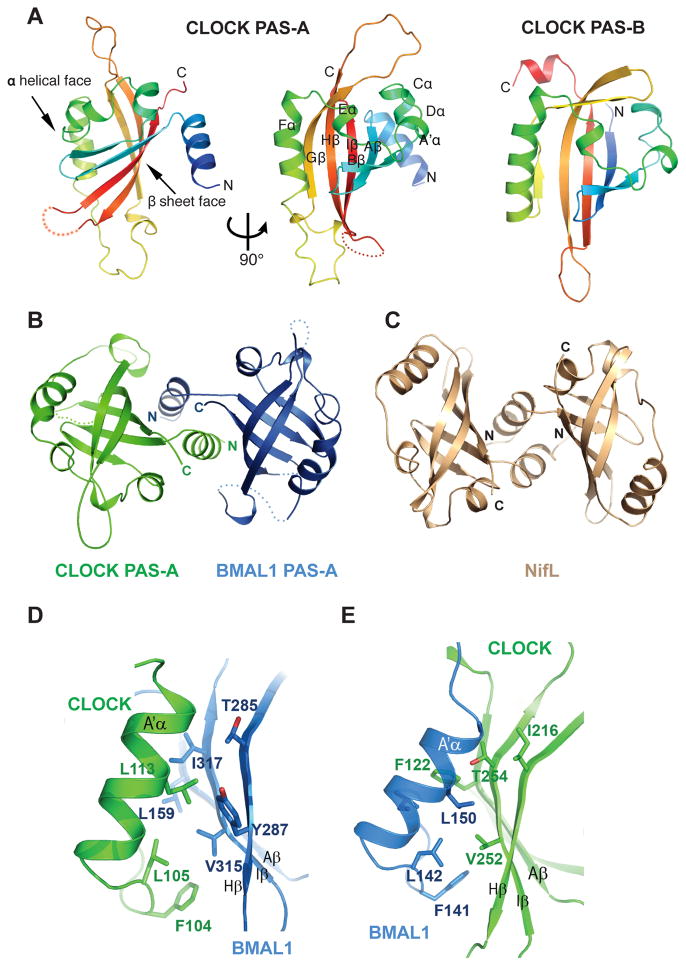
Fig. 2.
Structure and interaction of the PAS-A domains of CLOCK:BMAL1. (A) Ribbon representations of CLOCK PAS-A domain. Secondary structures are color ramped from blue to red and labeled from the A′α helix located N-terminal to the canonical PAS domain fold, in an alphabetical progression through the whole domain.. The CLOCK PAS-B domain is also shown for comparison. (B) Dimerization of the two PAS-A domains in CLOCK:BMAL1, looking down the approximate two-fold symmetry axis. (C) Similar domain-swapped structure of the redox sensing PAS domain of NifL from A. vinelandii (pdb: 2GJ3). (D) Left panel, detailed interface between A′α helix of CLOCK PAS-A (green) and the β sheet face of BMAL1 PAS-A (blue). Right panel, the corresponding interface between A′α helix of BMAL1 PAS-A and the β sheet face of CLOCK PAS-A.