Figure 7. Identification of CRY1 ubiquitination sites for FBXL3 and FBXL21.
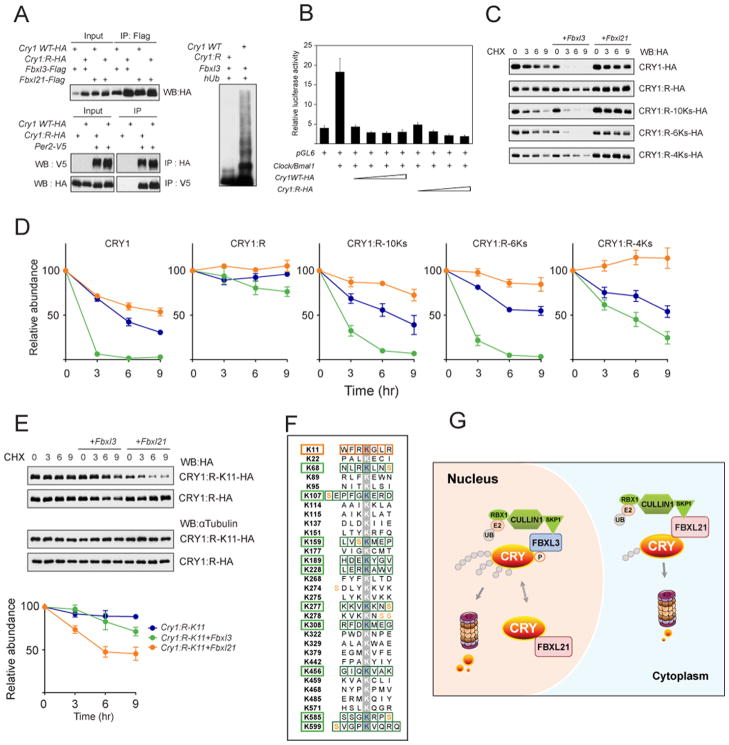
A. Left: CRY1:R co-immunoprecipited with FBXL3, FBXL21 and PER2. Upper: Co-immunoprecipitation of CRY1WT and CRY1:R with FBXL proteins. Lower: Reciprocal co-IP of WT and CRY1:R with PER2. WT or Cry1:R expression constructs were transfected into 293A cells as indicated (+). Right: 293A cells were co-transfected with indicated constructs. Whole-cell lysates were analyzed by western blotting with an anti-CRY1 antibody. Representative blots from 3 independent experiments are shown.
B. CRY1:R repressed CLOCK/BMAL1-mediated transcriptional activation of the Per2 promoter. pGL6 was transfected into 293A cells with the indicated constructs (+). Luciferase reporter assay showed similar repression efficiency for CRY1:R and CRY1 WT. Results are mean ± SEM for 3 independent experiments in duplicate.
C. Representative western blots of HA-tagged CRY1 (WT, R, R-10Ks, R-6Ks, or R-4Ks). The Cry1 construct was transfected alone or with Fbxl3 or Fbxl21 into 293A cells. Cells were treated with CHX (100 μg/ml) and collected after 0, 3, 6, or 9 hrs.
D. Quantification of CRY1 and CRY1 mutant degradation by Fbxl3 or Fbxl21 from 2 independent experiments in duplicate (n = 4 western blots; representative blots shown in C). Data represent mean ± SEM for expression of Cry1 alone (blue), Cry1 + Fbxl3 (green), or Cry1 + Fbxl21 (orange). Half life was determined by nonlinear, one phase exponential decay analysis. CRY1 half life: alone 5 hr; + Fbxl3 0.7 hr; + Fbxl21 9 hr. CRY1:R half life: alone >24 hr; + Fbxl3 21 hr; + Fbxl21 >24 hr. CRY1:R-10Ks half life: alone 6 hr; + Fbxl3 1.8 hr; + Fbxl21 21 hr. CRY1:R-6Ks half life: alone 8.9 hr; + Fbxl3 1.3 hr; + Fbxl21 >24 hr. CRY1:4Ks half life: alone 10.6 hr; + Fbxl3 4.6 hr; + Fbxl21 >24 hr. The half-life parameter, K, is significantly different in all conditions, p<0.0001, with the exception of the CRY1:R group.
E. FBXL21-mediated CRY1 degradation via the K11 residue. Representative western blots of Cry1:R-K11-HA, Cry1:R-HA are shown. The experiment was performed as in C. The K11 revertant underwent moderate degradation by FBXL21. K11 alone half life: >24 hr, + Fbxl3 18.4 hr, + Fbxl21 6.7 hr. The half-life parameter, K, for Fbxl21 is significantly different: p<0.0001).
F. 11 candidate CRY1 lysines subject to ubiquitination. All 31 lysine residues of CRY1 are shown, and candidate lysines for FBXL3- and FBXL21-mediated degradation are indicated with green and orange boxes, respectively.
G. Differential roles of FBXL21 in nuclear and cytoplasmic CRY turnover. FBXL21 appears to form SCF complexes only in the cytoplasm and functions as a cytoplasmic-specific, weak E3 ligase for CRY degradation. In contrast, nuclear FBXL21 antagonizes FBXL3-mediated CRY degradation, thus conferring a CRY-protective function. In the absence of FBXL21, cytoplasmic CRY is stabilized, whereas in the nucleus CRY is destabilized because FBXL21 cannot antagonize the action of FBXL3. See also Figure S6, S7.