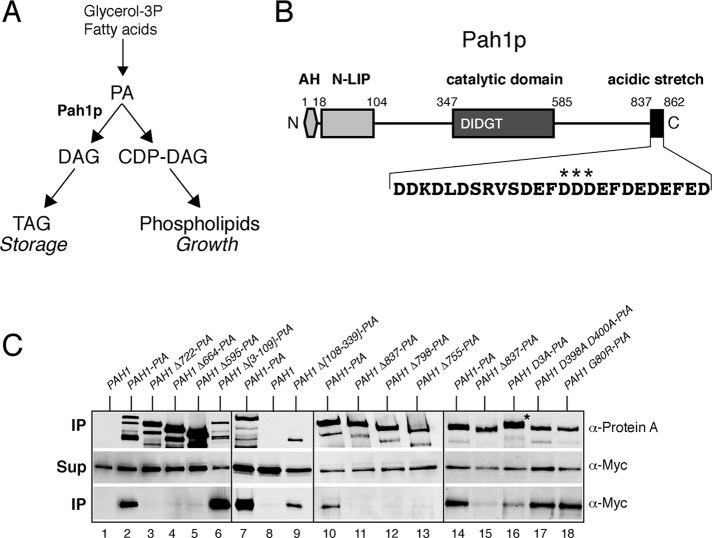
FIGURE 1:
The C-terminal acidic stretch of Pah1p is required for binding to the Nem1p-Spo7p complex in vivo. (A) Simplified schematic of the role of Pah1p in lipid biosynthesis. (B) Schematic of the primary structure of Pah1p with the position of key motifs/domains shown. AH, amphipathic helix. The sequence of the acidic stretch is shown, with the three aspartates mutated in Pah1D3A-PtA highlighted by stars. (C) Mutational analysis of the interaction of Pah1p and Nem1p-Spo7p. Control untagged PAH1 or the indicated wild-type or mutant PAH1-PtA fusions were expressed in pah1Δ cells carrying GAL-NEM1-Myc and GAL-SPO7. Cells were transferred from raffinose- into galactose-containing medium and grown for 8 h before lysis and affinity purification over IgG–Sepharose columns. Each of the four boxes corresponds to one independent representative set of experiments. Top and bottom (IP) correspond to equivalent amounts of affinity purified eluates, analyzed by SDS–PAGE (8%) and Western blotting using anti–protein A and anti-Myc antibodies, respectively. Middle (Sup) corresponds to equal amounts of clarified cell extracts used for the affinity purifications, analyzed by Western blotting using anti-Myc antibodies.