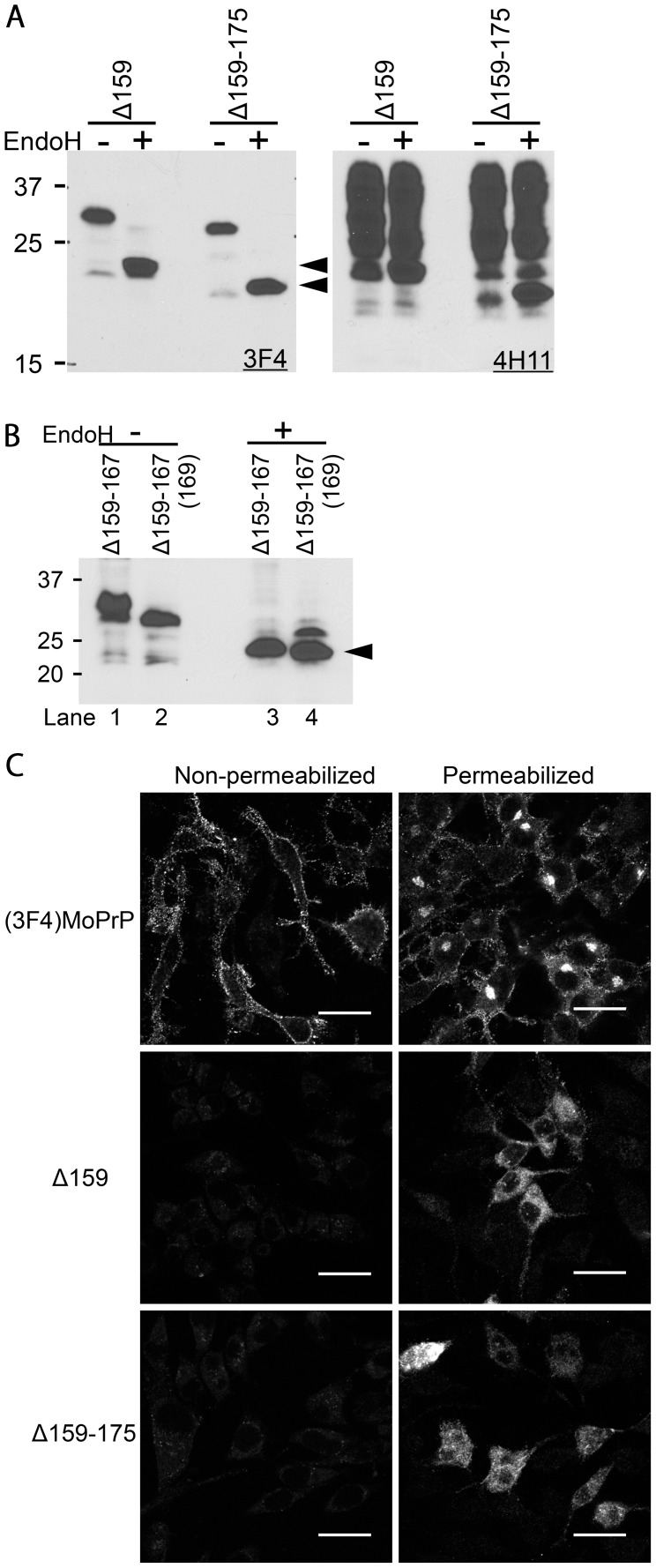
Figure 2. ΔPrPs are non-complex glycosylated and retained intracellularly.
A. N-linked glycans of ΔPrPs are not complex-type. Immunoblot comparing EndoH- and non-digested lysates of ΔPrP159 and Δ159–175 transfected cells. Left panel was probed with mAb 3F4 mAb, detecting transiently expressed ΔPrPs. On the right panel, the membrane was re-probed with mAb 4H11, detecting total PrP. Arrowheads depict deglycosylated ΔPrPs. B. The additional band of Δ159–167 represents a third N-glycan sensitive to EndoH. Upon EndoH digestion, all bands converge into a non-glycoform band (lane 3 and 4). In Δ159–167(169) the third glycosylation site is not present anymore. C. ΔPrPs are mainly distributed in intracellular compartments rather than on the cell surface. Confocal microscopy images of N2a cells transiently transfected with (3F4)MoPrP, Δ159 or Δ159–175, either permeabilized (right) or non-permeabilized (left) with 0.2% TX100 after fixation. Cells were immuno-labeled with mAb 3F4. (3F4)MoPrP-expressing cells show bright immunopositivity on the cell surface, whereas ΔPrPs are not observed on the cell surface. Also intracellular staining differs strongly between (3F4)MoPrP and ΔPrP expressing cells Scale bars, 25 µm.