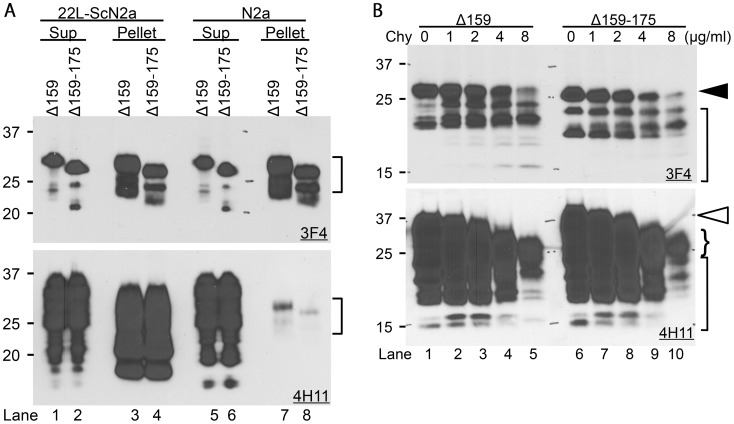
Figure 3. ΔPrPs spontaneously form aggregates that are detergent-insoluble and relatively chymotrypsin-resistant.
A. ΔPrPs are insoluble in detergent solution. Immunoblots showing partition of Δ159 and Δ159–175 in supernatant (Sup) and pellet fraction after ultracentrifugation of lysates supplemented with 4% sarcosyl. Upper panel is mAb 3F4, lower panel is membrane re-probed with mAb 4H11 detecting also endogenous wild-type PrP besides ΔPrPs. The majority of ΔPrPs is found in the insoluble fraction in both infected and non–infected cells (upper panel, lanes 3, 4, 7 and 8). Wild-type PrP almost completely partitions into the soluble fraction (Sup) in non-infected N2a cells with present conditions. PrP in pellet fractions of non-infected N2a cells is from ΔPrPs as can be seen from the banding pattern (Lane 7 and 8, square bracket). PrP in lanes 3 and 4 (lower panel) is PrP27-30. B. ΔPrPs are moderately resistant to chymotrypsin digestion. Immunoblots showing chymotrypsin-resistant fragments of Δ159 and Δ159–175 from transiently transfected N2a cell lysates digested with indicated concentrations of chymotrypsin (Chy). Note that full-length diglycoform of ΔPrP still remains at 8 µg/ml Chy (lanes 5 and 10, upper panel, closed arrowhead), whereas that of endogenous wild-type PrP is completely digested (lanes 5 and 10, lower panel, open arrowhead) and only small amounts of truncated fragments remained (curly bracket). Other smaller fragments in lane 5 and 10 (square-brackets) represent ΔPrPs.