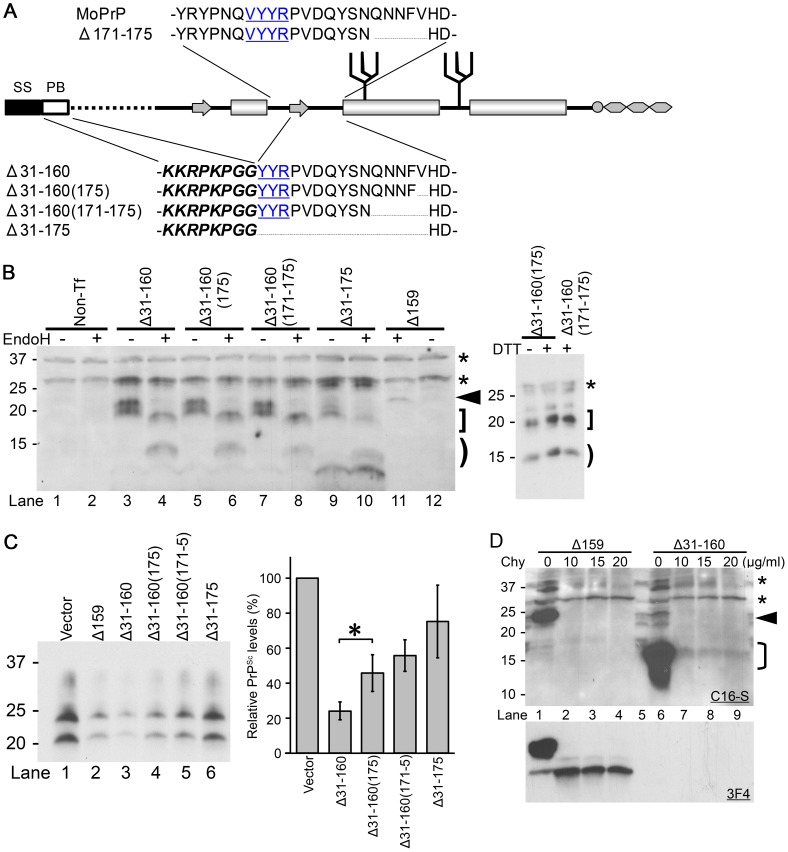
Figure 7. ΔPrPs lacking in addition the N-terminal region still exert efficient DNI.
A. Schematic illustration of Δ171–175, Δ31–160 and its variants. Δ31–160 variants lack the entire part between the polybasic motif (PB, italic and bold) and residue 161. SS, signal sequence. B. Δ31–160 variants are expressed at comparable levels and have EndoH-sensitive and –resistant N-glycans. Immunoblot comparing EndoH-digested and non-digested samples from N2a cells transiently transfected with Δ31–160 variants, probed with anti-PrP mAb C16-S raised against the C-terminal portion of H3. Non-Tf, samples prepared from N2a cells without transfection. Arrowhead denotes deglycosylated Δ159 (lane 11), bracket deglycosylated fragments, square bracket EndoH-resistant fragments. Asterisks indicate non-specific bands. Δ31–160 variants are not completely deglycosylated by EndoH, resulting in EndoH-resistant fragments (square bracket) which are observed also in reducing conditions (right panel; +/− dithiothreitol (DTT) treatment). Note that endogenous wild-type PrPc was not detected under used conditions. C. DNI of Δ31–160 variants is similarly inversely related to size of deletion and dependent on intact C-terminal H1–H2 portion. Immunoblot probed with mAb 3F4 showing PK-resistant (3F4)MoPrP co-transfected with indicated constructs into 22L-ScN2a cells (left panel). Δ31–160 was slightly more effective than Δ159. Right panel shows quantification of results as obtained from a triplicate experiment, using densitometry on ImageJ. Bars illustrate mean ± standard deviation. *, p<0.05. D. Δ31–160 forms spontaneous aggregates with moderate chymotrypsin (Chy) resistance. Immunoblots probed with mAbs C16-S (upper panel) or 3F4 (lower panel) for comparing Chy-resistant fragments of Δ31–160 with that of Δ159. Lysates from N2a cells expressing Δ159 or Δ31–160 were digested with indicated concentrations of Chy for 30 minutes and then digested with PNGaseF. A sample from non-transfected N2a cells is shown in the lane 5. Asterisks indicate non-specific C16-S bands. Arrowhead denotes deglycoform of Δ159, bracket deglycoform of Δ31–160.