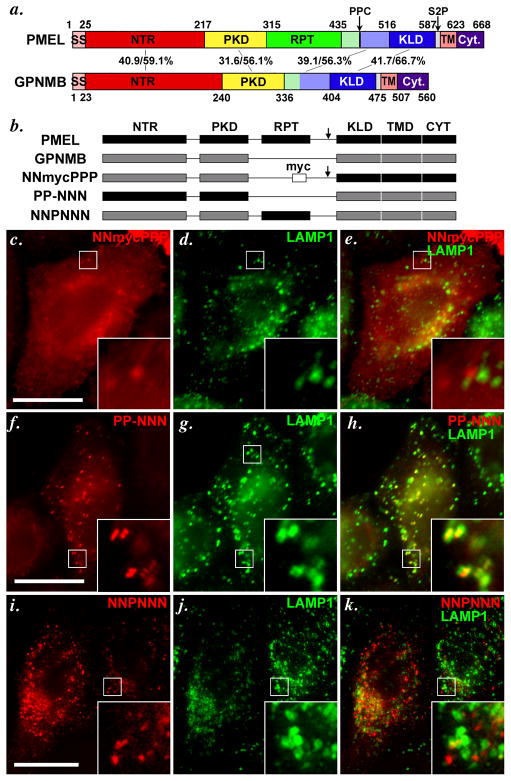
Figure 4. The lumenal NTR and PKD domains of PMEL and GPNMB confer distinct trafficking properties to chimeric constructs.
a. Schematic diagram depicting the indicated homologous subdomains within PMEL and GPNMB. GPNMB lacks a RPT domain, but contains NTR (N-terminal region), PKD (Polycystic Kidney Disease homology domain), KLD (Kringle-like domain), TMD (Transmembrane domain) and Cyt (cytoplasmic domain) regions homologous to those found in PMEL. Arrows indicate cleavage sites for a proprotein convertase (PPC) and site 2 protease (S2P) in PMEL. SS, signal sequence. Numbers in the middle correspond to the sequence identity/ similarity between human PMEL and human GPNMB within the indicated regions. Regions indicated in light green and light blue have been referred to as “GAP2” by Hearing and colleagues (Hoashi et al., 2006); for chimeric proteins, these regions were appended to the RPT and KLD domains, respectively. b. Schematic diagram of PMEL, GPNMB and chimeric proteins analyzed in c–k. The 6 subdomains in PMEL are indicated in black and named with a letter P (i.e., PPPPPP for PMEL), and 5 corresponding domains in GPNMB are indicated in gray and denoted with the letter N (e.g. NN-NNN for GPNMB; the missing RPT domain in GPNMB is denoted with a −). Arrow indicates presence of a PPC cleavage site; where indicated, a myc epitope tag has been inserted into GPNMB where the RPT domain would be in PMEL.. c–k. HeLa cells were transiently transfected with the indicated chimeric proteins, processed as in Figure 3, and analyzed by IFM-D for the transgene (red) and the lysosomal protein LAMP1 (green); a merged image is shown on the right, and insets show the boxed regions that are magnified 4X. Note that substitution of the NTR and PKD domains of PMEL with those of GPNMB (NNmycPPP) causes mislocalization of PMEL, as shown by loss of colocalization with the lysosomal marker LAMP1 (c–e). Conversely, the NTR and PKD domains of PMEL (PP-NNN, f–h), but not the RPT (NNPNNN, i–k), are sufficient to confer late endosome/ lysosome targeting to GPNMB, as shown by co-labeling with LAMP1. Bars, 20 μm.