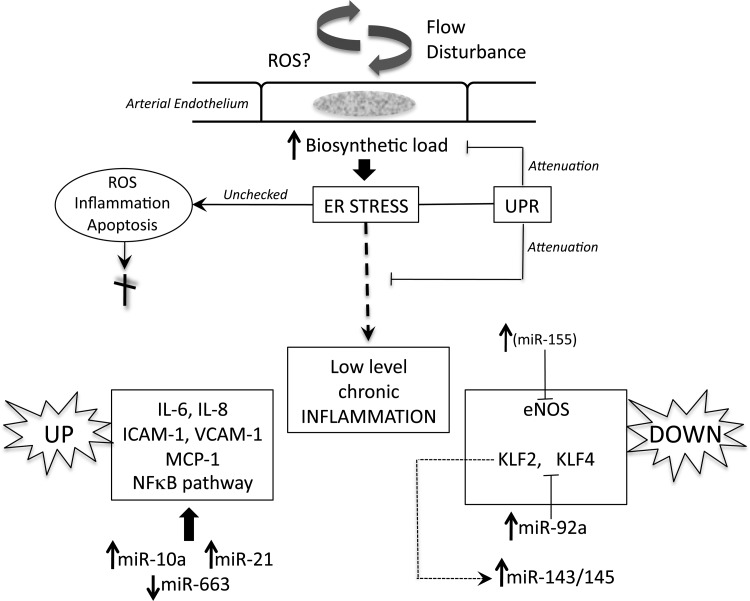
Figure 6.
A schema of regulatory mechanisms of endothelial atherosusceptibility discussed in the text. Flow disturbance in regions susceptible to atherogenesis places a stress load on the biosynthetic capacity of the endothelial endoplasmic reticulum (ER) that leads to ER stress. Activation of the unfolded protein response (UPR) compensates to maintain normal cell function but a residual low-level chronic inflammatory state persists. Pro-inflammatory molecules are up-regulated (left) and protective molecules down-regulated (right). MicroRNAs (miRs) regulated up or down by disturbed flow regulate (at least in part) the pro-inflammatory state and are shown in a reciprocal transcriptional relationship to their targets. Notes: eNOS is a direct target of miR-155, inhibiting eNOS in cytokine-induced inflammation, however, differential miR-155 expression has not yet been demonstrated in disturbed flow. miR-143/145 is increased by flow-induced suppression of KLF2 and is shed in endothelial exosomes (Figure 5D). Not shown in this schema or discussed in the text (because of space limitations) are other regulatory mechanisms believed to contribute to endothelial pro-inflammatory phenotype through the MAPK pathway that mimics cytokine-induced mechanisms and through a low level of protective Nrf2 in atherosusceptible endothelium in mice.17