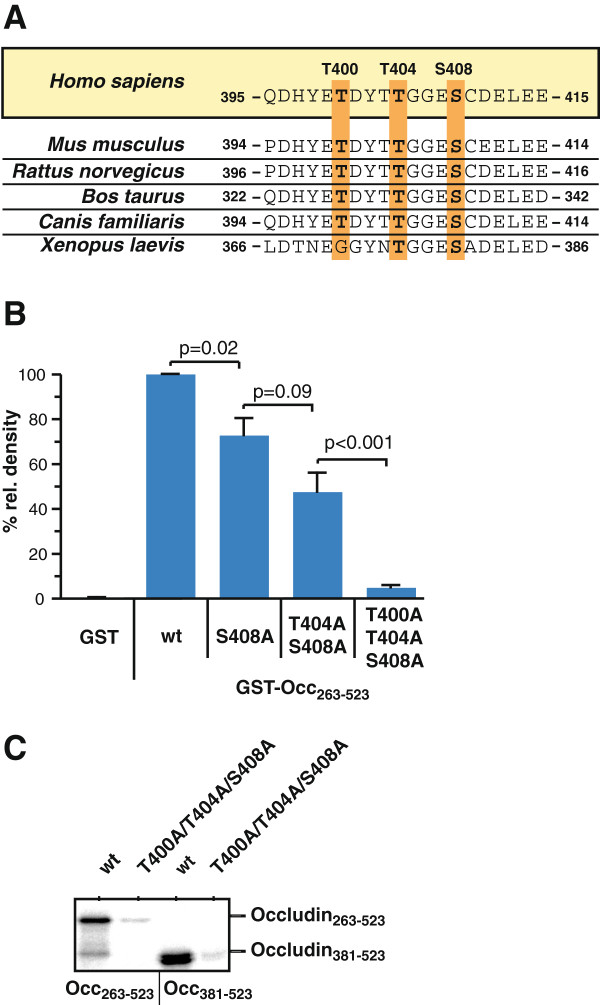
Figure 2.
CK2 phosphorylates a highly conserved T400/T404/S408 motif in the cytoplasmic tail of occludin. A) Alignment of amino acid sequences containing the CK2 phosphorylation motif in occludin from different species. Conserved threonine (T) and serine (S) residues targeted by CK2 are highlighted. B) Densitometric analysis of in vitro phosphorylation experiments using purified recombinant GST-fusion proteins of the occludin C-terminal domain and the indicated Ser/Thr to Ala mutated constructs. Data represent mean values +/− SEM of 4 independent experiments. C) Full-length cytoplasmic tail (GST-Occ263-523) and the C-terminal half of it (GST-Occ381-523) and the corresponding triple alanine mutated proteins were in vitro phosphorylated with recombinant CK2 and subsequently analyzed by autoradiography. The triple alanine mutations abrogated phosphorylation by CK2. The presented figure is a representative of 3 independent experiments.