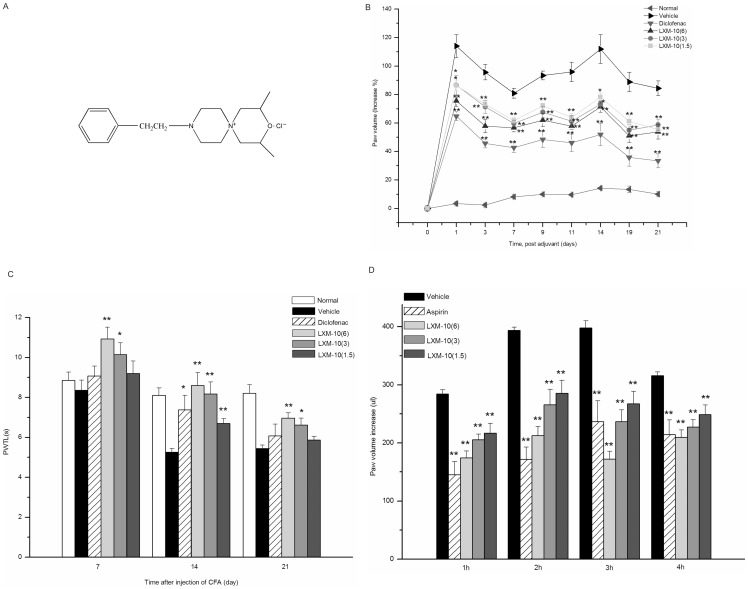
Figure 1. The chemical structure of LXM-10 and its anti-inflammatory effects in vivo.
(A) The chemical structure of the spirocyclopiperazinium compound LXM-10. (B) Effect of LXM-10 on complete Freund's adjuvant (CFA)-induced paw swelling. The rats were administered with LXM-10 (6, 3, 1.5 mg/kg, i.g.), diclofenac (5 mg/kg, i.g.) or vehicle for 21 days after injecting CFA. The paw volume was evaluated on days 0, 1, 3, 7, 9, 11, 14, 19 and 21. All data are mean ± SEM of 6 rats per group. Significant differences from the vehicle group at the same time are indicated by * P<0.05 and ** P<0.01. (C) Effect of LXM-10 on complete Freund's adjuvant (CFA)-induced thermal hyperalgesia. The rats were administered with LXM-10 (6, 3, 1.5 mg/kg, i.g.), diclofenac (5 mg/kg, i.g.) or vehicle for 21 days after injecting CFA. The thermal hyperalgesia were detected on days 7, 14, 21. All data are mean ± SEM of 6 rats per group. Significant differences from the vehicle group at the same time are indicated by * P<0.05 and ** P<0.01. (D) Effect of LXM-10 on carrageenan induced paw oedema. The rats were administered with LXM-10 (6, 3, 1.5 mg/kg, i.g.), aspirin (300 mg/kg, i.g.), or vehicle (i.g.) and the paw volume was measured at 1, 2, 3 and 4 h after challenged by carrageenan. All data are mean ± SEM of 8 rats per group. Significant differences from the vehicle group at the same time are indicated by * P<0.05 and ** P<0.01.