Abstract
Objective
Chronic asthma is characterized by ongoing recruitment of inflammatory cells and airway hyperresponsiveness leading to structural airway remodeling. Although α4β1 and β2 integrins regulate leukocyte migration in inflammatory diseases and play decisive roles in acute asthma, their role has not been explored under the chronic asthma setting. To extend our earlier studies with α4Δ/Δ and β2−/− mice, which showed that both a4 and b2 integrins have nonredundant regulatory roles in acute ovalbumin (OVA)-induced asthma, we explored to what extent these molecular pathways control development of structural airway remodeling in chronic asthma.
Materials and Methods
Control, α4Δ/Δ, and β2−/−mouse groups, sensitized by intraperitoneal OVA as allergen, received intratracheal OVA periodically over days 8 to 55 to induce a chronic asthma phenotype. Post-OVA assessment of inflammation and pulmonary function (airway hyperresponsiveness), together with airway modeling measured by goblet cell metaplasia, collagen content of lung, and transforming growth factor β1 expression in lung homogenates, were evaluated.
Results
In contrast to control and β2−/− mice, α4Δ/Δ mice failed to develop and maintain the composite chronic asthma phenotype evaluated as mentioned and subepithelial collagen content was comparable to baseline. These data indicate that β2 integrins, although required for inflammatory migration in acute asthma, are dispensable for structural remodeling in chronic asthma.
Conclusion
α4 integrins appear to have a regulatory role in directing transforming growth factor β-induced collagen deposition and structural alterations in lung architecture likely through interactions of Th2 cells, eosinophils, or mast cells with endothelium, resident airway cells, and/or extracellular matrix.
In acute asthma, dysregulated immunity triggers a Th2 response by antigen-presenting cells and Th2-derived cytokines, especially interleukin-4 (IL-4) and IL-13, promoting B-cell differentiation into immunoglobulin (Ig) E-sequestering plasma cells. Cross-linking of IgE receptors on mast cells releases histamines, prostaglandins, thromboxane, and leukotrienes, leading to bronchoconstriction, vasodilation, and mucus secretion [1]. Thus, a cascade of interactions between cells and soluble molecules in the airways results in bronchial mucosal inflammation and airway hyperresponsiveness (AHR) [2]. In chronic allergic asthma, there is continuous recruitment of Th2 as well as inflammatory cells in the lung and airways. These cells and their secreted products elicit structural changes in resident airway cells, including epithelial desquamation, goblet cell metaplasia, mucus hypersecretion, and thickening of submucosa, manifested as bronchoconstriction and AHR [3,4]. Prominent in the remodeling process is the thickening of the airway wall with development of subepithelial fibrosis from deposition of extracellular matrix proteins, such as collagen, laminin, fibronectin, and tenascin in the lamina reticularis beneath the basement membrane [5–11]. Despite the fact that the histologic features of airway remodeling in chronic asthma have been well-characterized, the immunologic and inflammatory mechanisms that maintain or enhance remodeling are incompletely understood.
Although mouse models of asthma do not completely reproduce all the hallmarks of human disease, and several pathophysiologic responses in mice have been of limited value in humans, these models have provided important insights into the pathophysiology of asthma and have been used for testing new treatments of allergic asthma [12]. Using mouse models, it was found that leukocyte migration into lung is an important early event in the pathogenesis of asthma, and it is mediated by a series of adhesive interactions between leukocytes and airway cells for which integrins (β2 and β1) have been found to be critical participants [13–18]. While CD18 (β2 integrin) null mice have been used to investigate the role of CD18 in allergic asthma [19], studies on α4 integrins have been previously limited to those using monoclonal antibodies or other inhibitors of α4 integrin [13–15,17,18,20,21].
Our recent studies with conditionally ablated α4 knockout mice tested in parallel with β2−/− mice showed that, while β2 integrins control inflammatory migration in the airways, α4 integrins subvert the onset of acute asthma by curtailing the initial sensitization process, as well as by preventing cross-talk between inflammatory leukocytes and their interaction with the endothelium and lung stroma [22]. Because chronic rather than acute asthma appears to be more relevant to human disease [23], it was important to explore the involvement of these two types of integrins in the chronic setting of allergen challenge. Thus, using a repeated-challenge protocol in a more chronic setting, we assessed, in these genetic mouse models, changes associated with structural remodeling of the airways to gain further insight into the contribution of α4 and β2 integrins to the airway remodeling in chronic allergic asthma.
Our data uncover novel information about the differential contribution of β2 vs α4 integrins in the composite phenotype of chronic asthma development and contribute to the understanding of mechanisms by which different cell subsets and molecular pathways participate in the pathophysiology and histopathology of chronic asthma.
Materials and methods
Animals
α4 integrinf/f mice were produced as described previously [24]. These mice were bred with Mxcre+ mice and the resulting Mxcre-+α4flox/flox mice were conditionally ablated as neonates by intraperitoneal injections of poly(I)poly(C) (Sigma Aldrich Co., St Louis, MO, USA) for interferon induction. cre−α4f/f mice were used as controls and the α4-ablated mice are referred to as α4Δ/Δand only mice with >95% α4 ablation in hematopoietic cells were used for studies. CD18 knockout mice were provided by Dr. Arthur Beaudet, Baylor College of Medicine (Houston, TX, USA). All animal protocols were approved by the University of Washington Institutional Animal Care and Use Committee. Mice were bred and maintained under specific pathogen-free conditions in University of Washington facilities and were provided with irradiated food and autoclaved water ad libitum.
Induction of chronic allergic asthma
Mice were sensitized and later challenged with ovalbumin (OVA; Pierce Biotechnology, Inc., Rockford, IL, USA) as described previously [25]. Briefly, mice were immunized with 100 μg OVA complexed with aluminium sulfate in a 0.2-mL volume, administered by intraperitoneal injection on day 0. On days 8 (250 μg OVA) and on days 15, 18, and 21 (125 μg OVA), mice anesthetized briefly with inhalation of isoflurane in a standard anesthesia chamber were given OVA by intratracheal administration. Intratracheal challenges were done as described previously [26]. Mice were anesthetized and placed in a supine position on a board. The animal’s tongue was extended with lined forceps and 50 μL OVA (in the required concentration) was placed at the back of its tongue. We have previously shown that this protocol results in increased AHR, inflammation of the airways, and Th2 cytokine production [25–27]. Prolonged inflammation was induced by subsequent exposure of mice to 125 μg OVA intratracheally three times a week until groups of mice were sacrificed on day 55 (chronic phase) after the last intratracheal challenge on day 54 (Fig. 1A). The control group [α4+/+cre− mice also injected with poly(I) poly(C)] received normal saline with aluminium sulfate by intraperitoneal route on day 0 and 0.05 mL 0.9% saline by intratracheal route on days 8, 15, 18, 21 and three times a week until they were sacrificed on day 55.
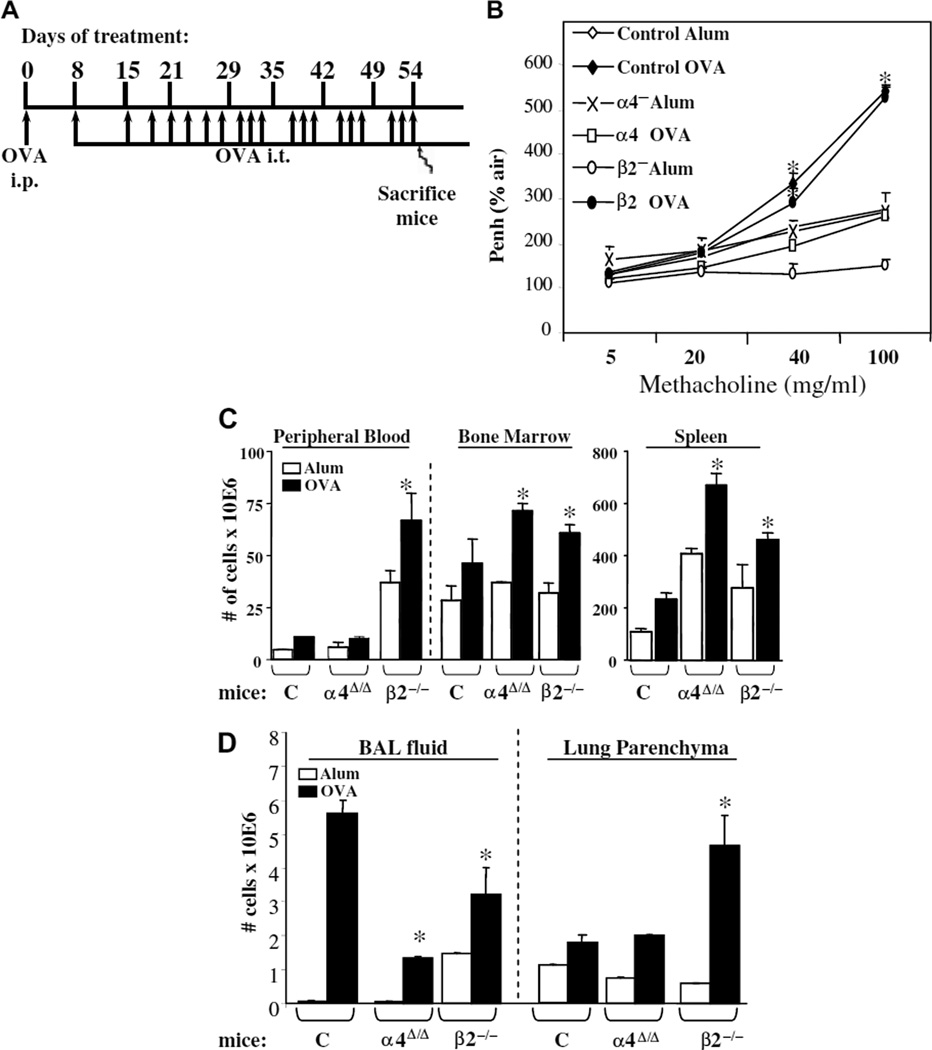
Figure 1.
(A) Diagram illustrating the experimental design for chronic asthma development. i.p. = intraperitoneal injection, i.t. = intratracheal instillation. (B) Lung function testing using whole-body plethysmography. Measurement of airway hyperresponsiveness at 24 hours after the last ovalbumin (OVA) challenge on day 54. The degree of bronchoconstriction to increasing doses of aerosolized methacholine was expressed as Penh (percentage of air as control). *p < 0.01 compared to baseline values in saline-treated control mice, n = 8 mice per group. Control mice, α4-deficient mice, and β2-deficient mice were treated with aluminium sulfate (alum) and OVA. Measurements of airway resistance by invasive plethysmography [22] were found to be in full agreement with Penh data. (C) Numbers of lymphoid and myeloid cells in blood (n/mL), bone marrow (n/femur), and spleen, and (D) total numbers of cells in bronchoalveolar lavage (BAL) fluid and lung parenchyma from the two genetic models and controls treated with alum or OVA. Data are from two independent experiments expressed as average ± standard error of the mean. n = 8 mice per group.
Bronchoalveolar lavage fluid
Mice underwent exsanguination by intraorbital arterial bleeding and then lavaged (0.4 mL three times) from both lungs. Total bronchoalveolar lavage fluid (BALf) cells were counted from a 50-μL aliquot and the remaining fluid was centrifuged at 200g for 10 minutes at 4°C and the supernatants frozen for assay of BALf cytokines later. Cell pellets were resuspended in fetal bovine serum and smears were made on glass slides. The cells, after air-drying, were stained with Wright-Giemsa (Biochemical Sciences, Inc., Swedesboro, NJ, USA) and differential counts enumerated using a light microscope at 40× magnification. Cell number refers to that obtained from lavage of both lungs/mouse.
Lung parenchyma cell recovery
Lung mincing and digestion were performed after lavage as described previously [17] with 100mg/mL collagenase for 1 hour at 37°C, and filtered through a no. 60 sieve (Sigma Aldrich Co.). All numbers mentioned in this article refer to cells obtained from one lung/mouse.
Lung histology
Lungs from other animals of the same group were fixed in 4% paraformaldehyde overnight at 4°C. Tissues were embedded in paraffin and cut into 5-μm sections. A minimum of 15 fields were examined by light microscopy. The intensity of cellular infiltration around pulmonary blood vessels was assessed by hematoxylin and eosin staining. Airway mucus was identified by staining with Alcian blue and periodic acid Schiff staining as described previously [22]. Subepithelial pulmonary fibrosis was detected by Masson’s trichrome and Martius scarlet blue stains as described in [28].
Lung immunohistochemical staining
Lungs were processed for immunohistochemical staining following standard procedure [26], then stained with either anti-vascular cell adhesion molecule-1 (VCAM-1; MK/2), anti-β1 (9EG7), or anti-transforming growth factor-β1 (TGF-β1). Briefly, tissues werefixed with 4% paraformaldehyde in 100 mM phosphate-buffered saline (PBS; pH 7.4) for 6 to 12 hours at 4°C, washed with PBS for 10 minutes three times, and then soaked in 10% sucrose in PBS for 2 to 3 hours, 15% sucrose in PBS for 2 to 3 hours, 20% for 3 to 12 hours at 4°C, and then embedded in OCT compound (Tissue-Tek 4583; Sakura Finetechnical Co., Ltd, Tokyo, Japan) and frozen in acetone-cooled dry ice. Frozen blocks were cut on a freezing, sliding microtome at 4 μm (LEICA CM1850 Cryostat) and air-dried for 30 minutes at room temperature (RT). After washing in PBS three times for 10 minutes at RT, 0.3% hydrogen peroxide was applied to each section for 30 minutes at RT to block endogenous peroxidase activity. Each slide was incubated with blocking solution to block nonspecific reactions, and appropriately diluted primary antibody was applied to each slide and incubated overnight at 4°C. After washing with PBS, slides were incubated with appropriately diluted specific biotin-conjugated secondary antibody solution for 1 hour at RT. After washing with PBS, slides were incubated in AB reagent for 1 hour at RT (ABComplex/ HRP; DAKO, Carpinteria, CA, USA), washed with PBS, and stained with 0.05% 3,3’-diaminobenzidine tetrahydrochloride; Sigma Aldrich Co.) in 0.05 M Tris buffer (pH 7.6) containing 0.01% H2O2 for 5 to 40 minutes at RT. Slides were counterstained with Mayer’s hematoxylin, dehydrated, and mounted.
Fluorescein-activated cell sorter (FACS) analysis
Cells from hemolyzed peripheral blood, bone marrow (BM), bronchoalveolar lavage, lung parenchyma, spleen, mesenteric lymph nodes, cervical lymph nodes, axillary lymph nodes, and inguinal lymph nodes were analyzed on a FACSCalibur (BD Immunocy-tometry Systems, San Jose, CA, USA) using the CELLQuest program. Staining was performed by using antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), peridinin chlorophyl protein (PerCP-Cy5.5), and Cy-chrome (PE-Cy5 and PE-Cy7). The following antibodies (BD Biosciences-Pharmingen, San Diego, CA, USA) were used for cell surface staining: APC-conjugated CD45 (30F-11), FITC-conjugated CD3(145-2C11), PE-Cy5 conjugated CD4 (RM4-5), PE-conjugated CD45RC (DNL-1.9), APC-conjugated CD8(53-6.7), PE-Cy5 conjugated B220 (RA3-6B2), FITC-conjugated IgM, PE-conjugated CD19 (ID3), PE-conjugated CD21(7G6), FITC-conjugated CD23 (B3B4), APC-conjugated GR-1 (RB6-8C5), and PE-conjugated Mac1(M1/70). PE-Cy5 conjugated F4/80 (Cl:A3-1[F4/80]) was obtained from Serotec Ltd. (Raleigh, NC, USA). PE-conjugated anti-α4 integrin (PS2) and anti-VCAM-1 (M/K-2) was from Southern Biotechnology (Birmingham, AL, USA). Irrelevant isotype-matched antibodies were used as controls. Among hematopoietic cells, CD45+/ CD3+ were T cells, CD3+/CD4+ were helper T cells, and CD3+/CD8+ were cytotoxic T cells. B cells were B220+. Gr-1+/F4/80− cells were granular cells (e.g., neutrophils, eosinophils) and Gr-1−/F4/80hi cells were tissue macrophages.
Cytokines
Cytokines (IL-2, IL-4, IL-5, tumor necrosis factor-α [TNF-α], and interferon-γ [IFN-γ]) in BALf and serum were assayed by FACS with Mouse Th1/Th2 Cytokine Cytometric Bead Assay (BD Biosciences) following manufacturer’s protocol. Manufacturer’s sensitivity for IL-2, IL-4, and IL-5 is 5 pg/mL, for IFN-γ is 2.5 pg/mL, and for TNF-α is 6.3 pg/mL. IL-13 and eotaxin were measured by enzyme-linked immunosorbent assay (ELISA) using Quantikine M kits (R&D Systems, Minneapolis, MN, USA) and the limit of detection is 1.5pg/mL for IL-13 and 3 pg/mL for eotaxin.
OVA -specific IgE and IgG1 in plasma
Anti-mouse IgE (R35-72) and IgG1 (A85-1) from BD Biosciences were used for measuring OVA-specific IgE and IgG1, respectively, by standard ELISA procedures as described previously [29]. The lower and upper limits of detection for IgE and IgG1 are 3 ng/mL to 10 ug/mL (minimal detectable dose determined by adding 2 standard deviations of the mean OD 405 nm value for 20 replicates of the zero standard and calculating the corresponding concentration.)
Pulmonary fibrosis
Martius scarlet blue and Masson’s trichrome stains in paraffin lung sections were used to visualize lung fibrosis [28].
Soluble VCAM-1 and soluble collagen in lung homogenate
Soluble VCAM-1 was determined as described previously [22]. Total amount of soluble collagen in the lung was measured using a Sircol collagen assay kit from Biocolor (Newtownsbury, Northern Ireland, UK), according to the method described [26]. In all experiments, a collagen standard was used to calibrate the assay.
Lung function testing
In vivo AHR to methacholine was measured 24 hours after the last OVA challenge in conscious, free-moving, spontaneously breathing mice using whole-body plethysmography (model PLY 3211; Buxco Electronics, Sharon, CT, USA) as described previously [25]. Mice were challenged with aerosolized saline or increasing doses of methacholine (5, 20, and 40 mg/mL) generated by an ultrasonic nebulizer (DeVilbiss Health Care, Somerset, PA, USA) for 2 minutes. The degree of bronchoconstriction was expressed as enhanced pause, a calculated dimensionless value, which correlates with the measurement of airway resistance, impedence, and intrapleural pressure in the same mouse. Penh readings were taken and averaged for 4 minutes after each nebulization challenge. Penh was calculated as follows:
Penh = [(Te/Tr − 1) × (PEF/PIF)]
where Te is expiration time, Tr is relaxation time, PEF is peak expiratory flow, and PIF is peak inspiratory flow × 0.67 coefficient. The time for the box pressure to change from a maximum to a user-defined percentage of the maximum represents relaxation time. Tr measurement begins at the maximum box pressure and ends at 40%.
Th differentiation, intracellular staining, and ELISA assay for IL-17A and IFN-γ
Cell suspensions from spleen were enriched for CD4+ T cells using anti-CD19 and anti-CD8 antibodies and BioMag goat anti-rat IgG Fc beads (Qiagen Inc, Valencia, CA, USA). Naïve CD4+ CD62L+ cells were sorted by flow cytometry and α4+ cells were excluded from α4Δ/Δ cells (4.1%). Five-hundred thousand naı¨ve CD4+ CD62L+ T cells were cultured under Th0 conditions: anti-CD3 [1 mg/mL] and anti-CD28 [2 mg/mL]; or Th17 polarization conditions: anti-CD3 [1 mg/mL], anti-CD28 [2 mg/mL], IL-6 [10 ng/ mL], (Peprotech, Rocky Hill, NJ, USA), TGF-β [5 ng/mL], (Peprotech), anti-IL-4 [5 mg/mL] (clone 11B11) and anti-IFN-γ [5 mg/ mL] (clone XMG1.2); or inducible regulatory T-cell conditions: anti-CD3 [1 mg/mL], anti-CD28 [2 mg/mL], TGF-β [5 ng/mL], anti-IL-4 [5 mg/mL], anti-IFN-γ [5 mg/mL], and IL-2 [100 U/ mL] (eBioscience, Inc., San Diego, CA, USA). After 4 days, cells were restimulated with phorbol myristate acetate (PMA) (50 ng/ mL; Sigma Aldrich) and ionomycin (1 mg/mL; Sigma Aldrich) and monensin (2 μM; eBioscience) for 5 hours. Cells were stained with CD4-FITC and/or CD103-PE. Intracellular staining was performed using Perm and Fix solutions from eBioscience, anti-IL-17A-PE (clone eBio17B7), anti-IFN-γ-APC (clone XMG1.2), and anti-Foxp3-FITC. These antibodies were obtained from eBioscience. For ELISA analysis, supernatants were harvested after 4 days. IL-17A and IFN-γ levels were analyzed using monoclonal antibodies and recombinant cytokine standards from eBioscience. Detection limits were IL-17A (125 pg/mL) and IFN-γ (100 pg/mL).
Statistics
Mean ± standard error of the mean was calculated using Student’s t-test in Excel Software (Microsoft, Redmond, WA, USA). A p value < 0.01 was considered statistically significant.
Results
α4Δ/Δ mice fail to develop AHR to chronic airway challenge by allergen
To determine pulmonary function in the OVA-treated mice, we measured AHR to increasing doses of methacholine by noninvasive whole-body plethysmography (Penh) (Fig. 1B). While both post-OVA control and β2−/− mice show significantly increased Penh value over saline-treated controls at 40 and 100 mg/mL methacholine, OVA-treated α4Δ/Δ mice have a Penh value similar to baseline values from untreated or saline-treated mice. Using the same genetic models (α4Δ/Δ, β2−/−), we have previously shown [22] that measurements of airway resistance by invasive plethysmography were in full agreement with responses by noninvasive whole-body plethysmography, as measured here.
Migration of leukocytes from circulation to lung and to airways
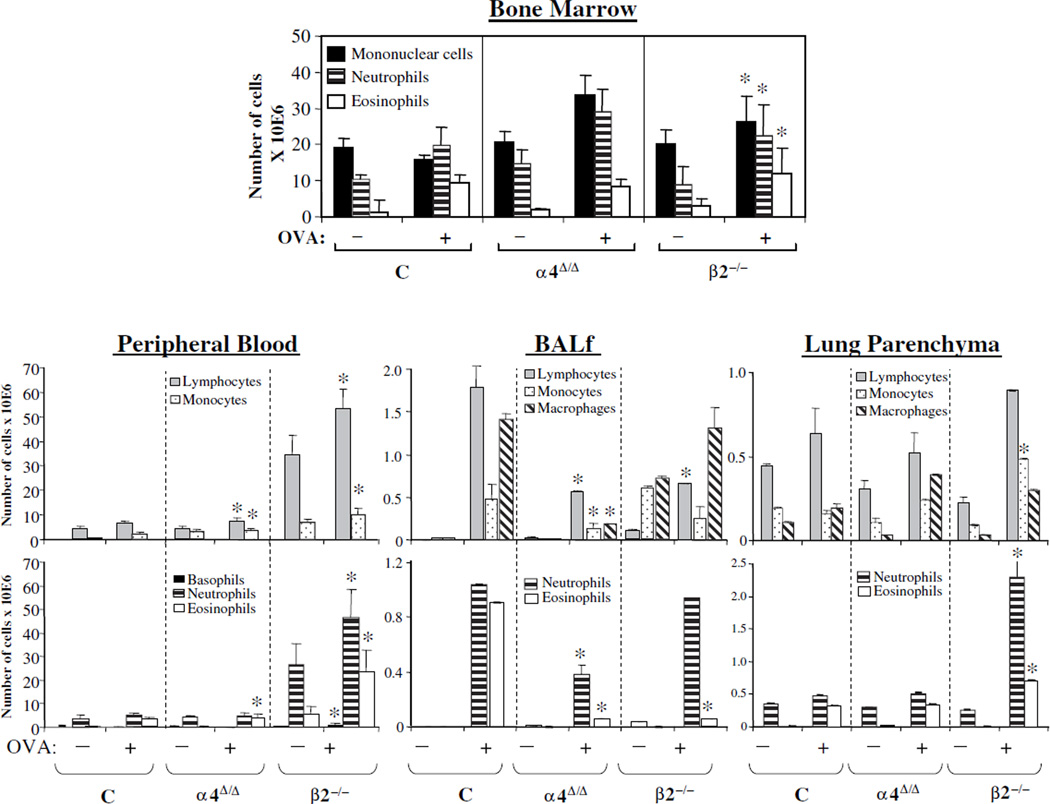
Total number of lymphoid and myeloid cells present in BM, peripheral blood circulation, spleen, or in BALf and lung parenchyma were assessed through measurements of total nucleated cell counts (Fig. 1C and Fig. 1D) and their differentials were determined by FACS based on specific antigen expression and by cell morphology from smears (Fig. 2 and Suppl. Table 1). Both total cell numbers and differentials varied among the animal groups and tissues studied. It is worth noting that differences among the groups also exist at baseline (i.e., increase in leukocytes in peripheral blood and spleen), as previous studies with these mice indicate [19,24]. In BM and peripheral blood, the total number of different leukocyte subsets was higher post-OVA in β2−/− mice compared to controls. α4Δ/Δ mice also had higher levels than controls in BM, with one exception, i.e., eosinophils. In lung parenchyma, β2−/− mice again had the highest numbers of leukocytes, reflecting both recruitment and accumulation in lung. In BALf, all leukocyte subsets were low in α4Δ/Δ mice, whereas only lymphocytes and eosinophils were reduced in β2−/− mice (Fig. 2) compared to controls. It is of interest that, as in acute asthma, eosinophils were not increased in BALf of both β2−/− and α4Δ/Δ mice, suggesting that for the interstitial airway migration of these cells, β2 and α4 are both important and nonredundant. However, chronic accumulation of eosinophils in lung parenchyma is α4- and β2-integrin–independent. Nevertheless, despite minimal eosinophil presence in BALf and increased presence of eosinophils in lungs of both genetic models, AHR was induced in β2−/− mice only, but not in α4Δ/Δ mice.
Figure 2.
Total numbers of all leukocytes in bone marrow, peripheral blood, bronchoalveolar lavage fluid (BALf), and lung parenchyma in control, α4-deficient (α4Δ/Δ), and β2−/− mice pre- and post-chronic ovalbumin (OVA) treatment. Total numbers (× 10E6) of cells were counted in a Beckman Coulter Counter and differentials (neutrophils, eosinophils, monocytes, etc.) were assessed by fluorescein-activated cell sorting (specific antigen expression) and morphology in smears. *p < 0.01 from control values post-OVA, n = 8 mice per group, C = Control mice.
Inflammation and fibrosis in lungs in response to chronic OVA challenge
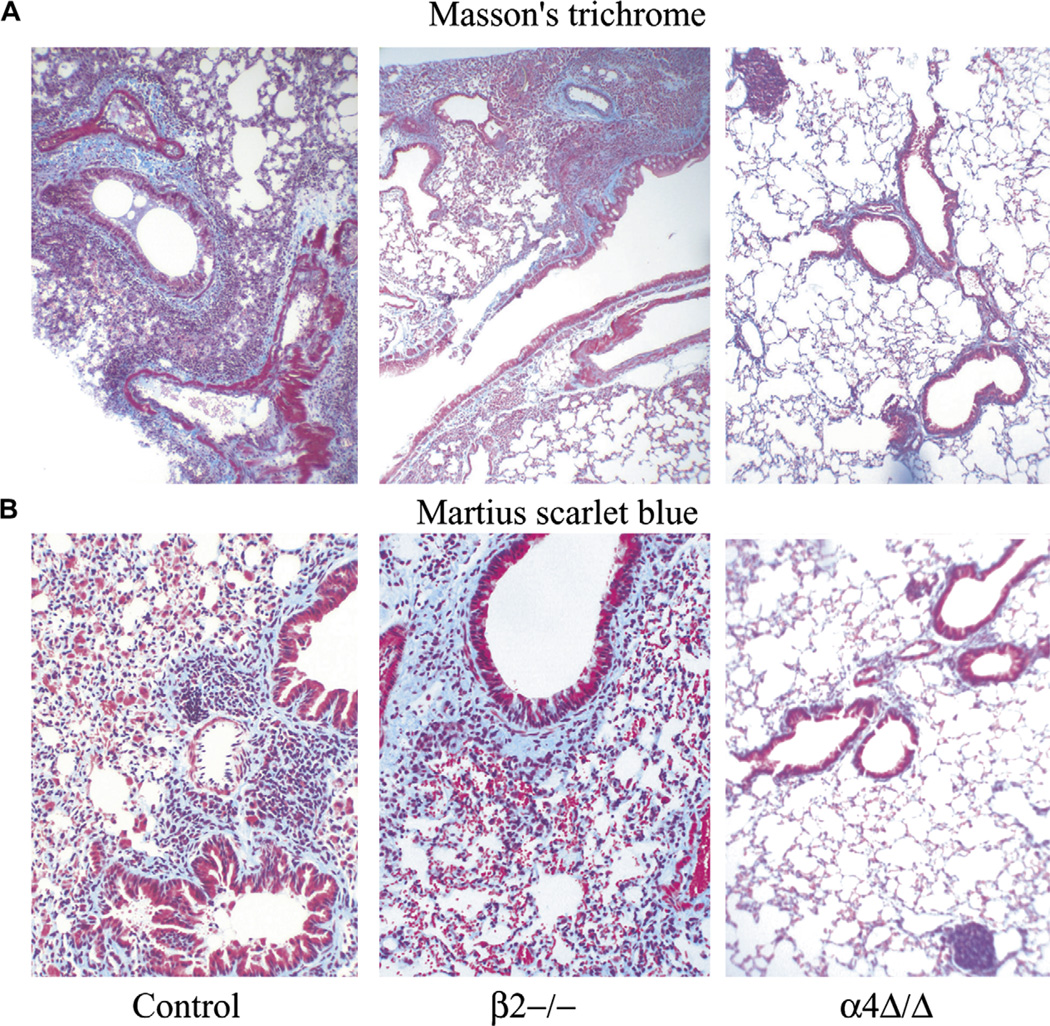
To assess the degree of inflammation and structural fibrotic remodeling in response to chronic allergen challenge, paraffin-embedded lung sections from untreated and OVA-treated mice were stained with hematoxylin and eosin to detect inflammatory cell migration into lung, Alcian blue to detect goblet cell metaplasia and mucus secretion in the luminal spaces of the lung and Masson’s trichrome and Martius scarlet blue to assess changes primarily in lung collagen content (Fig. 3). While OVA-treated control and β2−/− lungs show considerable inflammatory changes and increased deposition of collagen, indicating subepithelial fibrosis compared to aluminium sulfate-treated mice, in OVA-treated α4Δ/Δ mice no significant changes from non-OVA-treated control mice were noted. Mononuclear cell accumulation in lungs of α4Δ/Δ mice was frequently in a nodular pattern within the parenchyma (Fig. 3, right panel), unlike the other models.
Figure 3.
Paraffin sections of lungs from ovalbumin-treated mice were stained with (A) Masson’s trichrome stain revealing the collagen depositions around the airways and (B) Martius scarlet blue for collagen and fibrin deposition in interstitial spaces of the parenchyma. Adjustments for brightness, contrast, and color balance using Adobe Photoshop were made in order to match in all six photographs.
Th2/Th1 cytokines in BALf and plasma and IgE and IgG1 levels in plasma
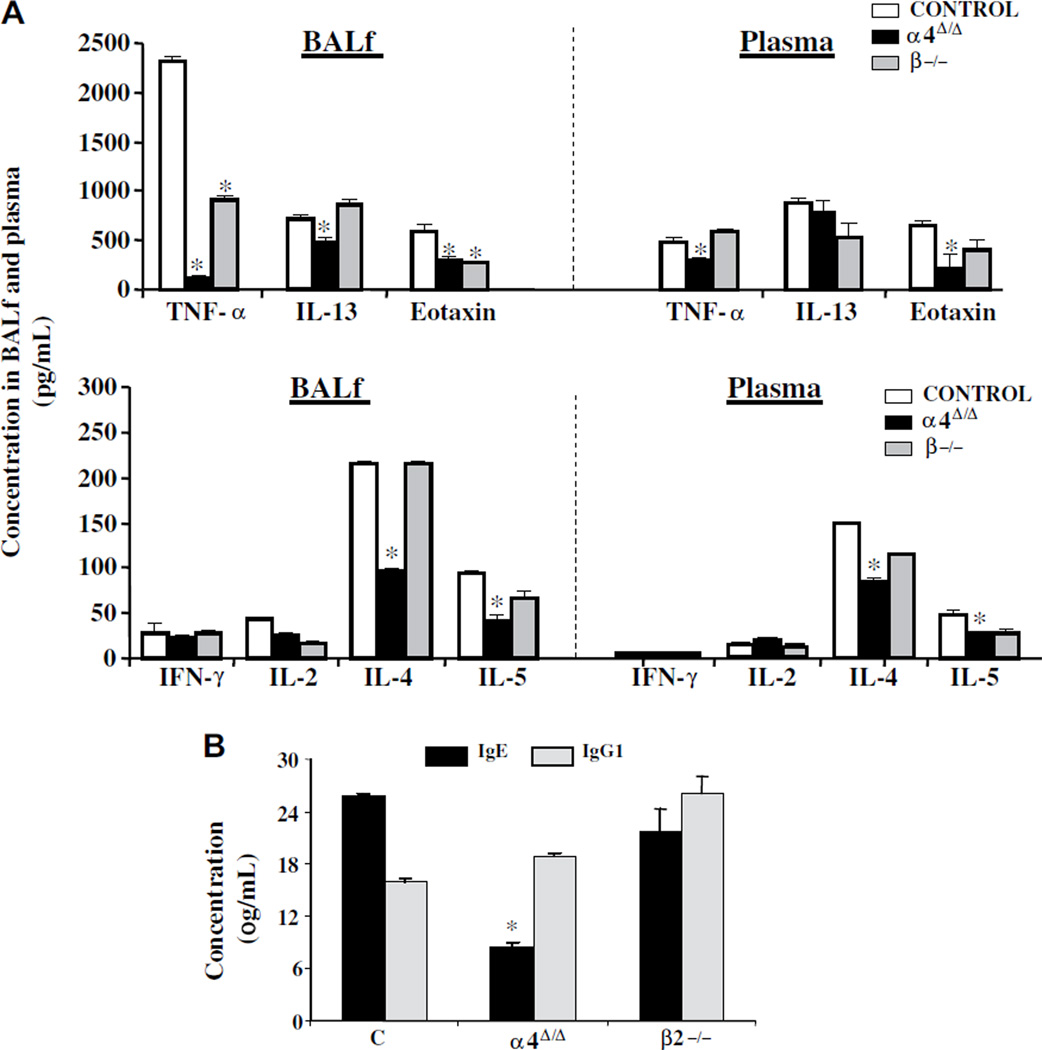
Levels of Th1 and Th2 cytokines and eotaxin were measured in plasma and BALf as described in Materials and Methods. All Th2 cytokines (i.e., IL-4, IL-5, IL-13), eotaxin, and TNF-α levels were low in BALf (Fig. 4A, left panels) of α4Δ/Δ mice compared to both control and β2 mice post-OVA, whereas in plasma (Fig. 4A, right panels), IL-13 levels were similar to other groups. β2−/− mice developed high levels of cytokines in circulation, like control mice, but in BALf (Fig. 4), only TNF and eotaxin were significantly lower than controls (see Suppl. Table 2), commensurate with the lower eosinophil levels (Fig. 2). Furthermore, OVA-sensitization responses were largely attenuated in α4Δ/Δ mice in contrast to the other two groups, as indicated by levels of OVA-specific IgE in plasma (Fig. 4C), corroborating previous findings in acute asthma [22].
Figure 4.
(A) Concentration of cytokines (pg/mL) post-ovalbumin (OVA) (upper two panels) in bronchoalveolar lavage fluid (BALf) and plasma in control (white bars), α4Δ/Δ (black bars), and β2−/− (gray bars) mice (*p < 0.01 compared to post-OVA control), and (B) concentrations of OVA-specific immunoglobulin (Ig) E and IgG1 (μg/mL) in plasma of OVA-treated mice in the three genotypes. Data (see Suppl. Table 2) are averaged from two independent experiments, all assays were run in triplicate, n = 8 mice per group, and *p < 0.01 compared to post-OVA control values. IFN-γ = interferon-γ; IL = interleukin; TNF-α = tumor necrosis factor-α.
Soluble VCAM-1 in BALf and plasma and VCAM-1 expression in lung
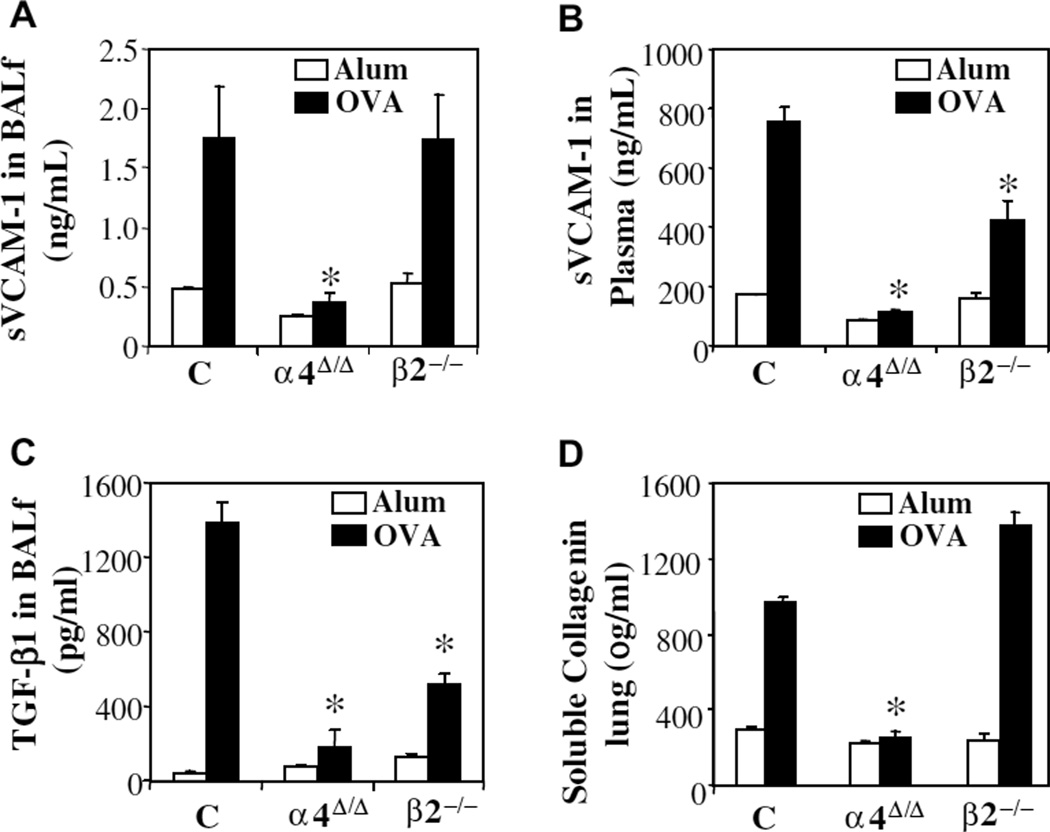
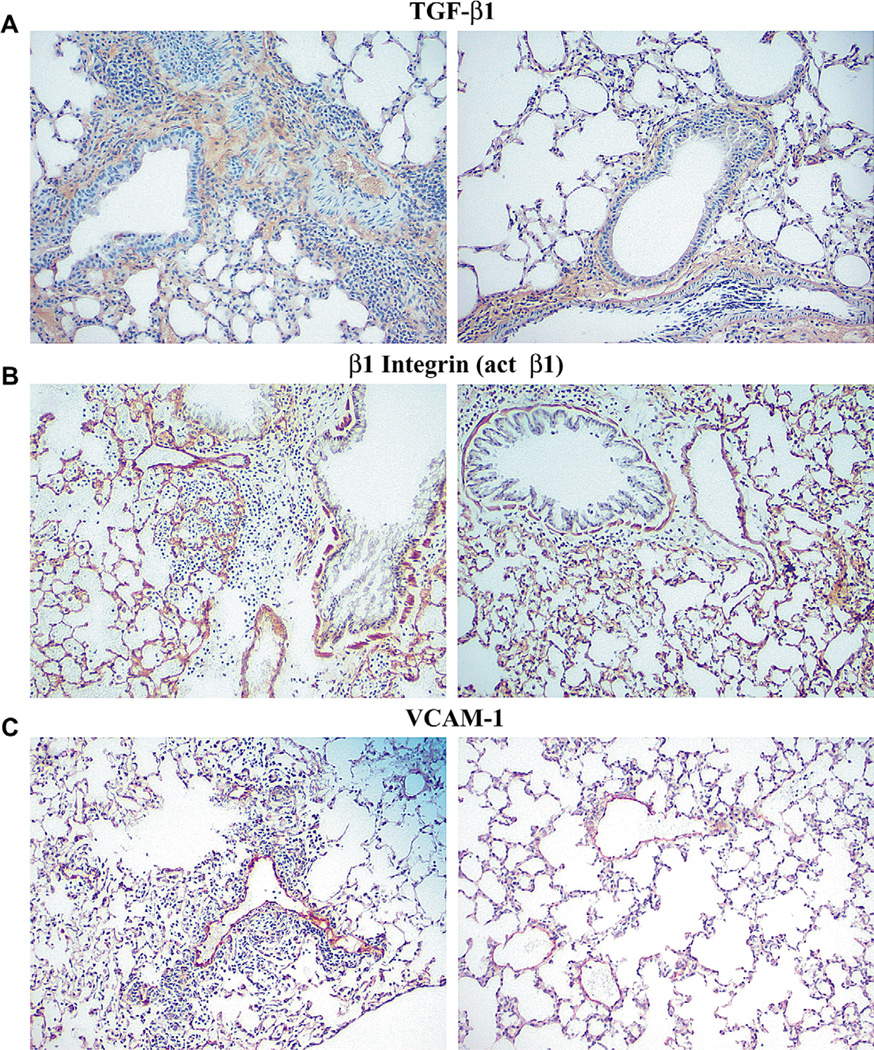
Because increases in cytokine levels (e.g., IL-4, IL-13, TNF-a) upregulate expression of VCAM-1 and increased VCAM-1 is a hallmark of allergic asthma phenotype, we next investigated whether the presence or absence of asthma phenotype affected the levels of soluble VCAM-1 in plasma and BALf or its expression in the lung in the two genetic models compared to controls. Soluble VCAM-1 levels post-OVA in both plasma and BALf were increased in control and β2−/− mice, but in α4Δ/Δ mice were quite low by comparison (Fig. 5A and Fig. 5B). Histochemical evidence of VCAM-1 protein in lung was also increased in response to OVA in both control and β2−/− lung (Fig. 6C, left panel), similar to changes seen in acute asthma [22]. However, in α4Δ/Δ lung post-OVA expression of VCAM-1 (Fig. 6C, right panel) was almost unchanged from baseline and lower than what was observed in the other two groups post-OVA. Therefore, although reduced levels of relevant cytokines could be responsible for decreased upregulation of VCAM-1 in α4Δ/Δ mice, other reasons, e.g., unresponsive endothelium in α4Δ/Δ mice, cannot be excluded.
Figure 5.
(A) Soluble vascular cell adhesion molecule-1 (sVCAM-1) concentrations (ng/mL) in bronchoalveolar lavage fluid (BALf) and (B) in plasma measured by enzyme-linked immunosorbent assay (ELISA), (C) transforming growth factor-β1 (TGF-β1) levels (pg/mL) in BALf measured by ELISA, and (D) soluble collagen content (μg/mL) of lung measured by Sircol dye kit. Data are averaged from two independent experiments ± standard error of mean. All assays were run in triplicate, n = 8 mice/group, and *p < 0.01 compared to post-ovalbumin (OVA) control values.
Figure 6.
Lung tissue sections, stained for transforming growth factor-β1 (TGF-β1) (anti-TGF-β1), 9EG7 (activated anti-β1 antibody), and vascular cell adhesion molecule-1 (VCAM-1) (anti-VCAM-1, MK/2) as described in Materials and Methods. (A) Images from Control (left) and α4Δ/Δ lung (right) post-ovalbumin (OVA) treatment and challenge stained with anti-TGF-β1. (B) Images of β2−/− lung (left) and α4Δ/Δ lung (right) post-OVA stained with 9EG7. (C) Images of β2−/− lung (left) and α4Δ/Δ lung (right) post-OVA stained with anti-VCAM-1. There were no significant differences in labeling with 9EG7, but labeling for TGF-β and VCAM-1 was less intense in α4Δ/Δ mice.
TGF-β1 and soluble collagen in BALf
TGF-β1 has been thought to be instrumental in fibrosis development and consequent collagen deposition in lung. Therefore, because subendothelial collagen deposition was present in control and β2−/− mice by immunohistochemistry (Fig. 3), we measured TGF-b1 in BALf (Fig. 5C) and soluble collagen levels in lung (Fig. 5D) of these mice. Significant increases in TGF-β1 and soluble collagen from aluminium sulfate-treated mice were only seen in control and β2−/− mice post-OVA. In contrast, in α4Δ/Δ mice, levels were very low consistent also with immunohistochemical documentation of TGF-β1 in lung sections (Fig. 6A, right panel).
Discussion
Although a body of literature is available describing the development of acute asthma in animal models, data comparing asthma and airway remodeling in both acute and chronic asthma settings by employing the same genetic mouse models are rare. As chronic asthma is considered more relevant to the human disease, molecular pathways involved in its development are important for designing targeted therapies [12,30]. Changes in chronic asthma are perpetuated because of a continuous dialogue between inflammatory cells and resident cells and matrix components in airways. The preferential recruitment of effector cells (eosinophils, Th2 cells) in the lung and airways is mediated by a cascade of adhesive interactions initiated by activated endothelial cells. However, the distinct molecular pathways that dominate these processes are continuously being revised. Studies with antifunctional antibodies yielded conflicting data, depending on the type, dose and route of administration of the antibody, or on the animal model used [13–18,20,21,31,32]. Data with CD18-deficient mice suggested an absolute requirement of CD18 for recruitment of eosinophils in the airways and for AHR [19]. Our parallel comparison of CD18 with conditional α4Δ/Δ mice in acute asthma showed that, in the latter, not only was AHR and migration of cells to airway lumen prevented, as in the CD18−/− mice, but there was attenuation of the sensitization process, minimal recruitment of eosinophils and lymphocytes in the lung parenchyma, and no upregulation in VCAM-1 expression [22]. As reported here, attenuation of all features of acute asthma observed in the absence of α4 integrins were, by and large, maintained during the chronic allergen challenge, although there was a higher leukocyte accumulation in the lungs of chronically challenged α4Δ/Δ mice (Fig. 1D and Fig. 2). Surprisingly, however, and in contrast to acute asthma, absence of β2 integrins (CD18) did not prevent chronic asthma development or its accompanied structural changes and AHR, despite the fact that migration of eosinophils to airways (BALf) (Fig. 1D and Fig. 2) was restrained in these mice. Although one cannot theoretically exclude the possibility that eosinophil degranulation differences among the two genetic models influence the cell numbers recorded, there is no experimental evidence supporting this notion.
Thus, the migratory flow of eosinophils from the systemic circulation to lung and then through the lung interstitium to airway lumen may be dictated by differences in adhesive interactions between pulmonary vasculature and that of systemic bronchial circulation. Our data highlight the fact that α4 and β2 integrins play critical but nonredundant roles in facilitating this pathway of inflammatory cell migration from lung interstitium to airway lumen. Cooperativity between α4β1 (ligating domains 1 and 4 of VCAM-1) and αMβ2 (recognizing only domain 4 of VCAM-1) in mediating adhesion and release from luminal ligands [23] may provide a mechanistic basis for such an outcome. However, the fact that the asthma phenotype was not prevented in VCAM-1-deficient mice [22] suggests that VCAM-1 upregulation and its interaction with integrins is not of critical importance in this process and that ligands other than VCAM-1 in airways and lung (i.e., E-selectin or fibronectin) may play pivotal roles in the absence of VCAM-1.
Total cell and total eosinophil accumulation in lung was high in β2−/− mice, even higher than in control mice. Such a high accumulation of inflammatory cells in lung, not necessarily in BALf, may be contributing to chronic asthma development in these mice. However, our data with α4Δ/Δ mice would argue that mere accumulation of inflammatory cells, including eosinophils, in lung might be necessary but not sufficient for asthma development. It is possible that the state of activation of accumulated cells is more important than their presence. For example, α4Δ/Δ mice did not show the elevated levels of TGF-β1, presumably because in the absence of α4 integrins, activation of the latent TGF-β1 in eosinophils (by mast-cell tryptase or αvβ6 on epithelia [4]) was not induced [33]. Furthermore, in vitro studies have previously implicated α5β1 expressed by airway smooth muscle cells in regulating fibronectin deposition after TGF-β1 stimulation [34]. Thus, although the α5β1-dependent pathway is intact in α4Δ/Δ mice, the low levels of TGF-β1 are consistent with reduced fibrin deposition seen in α4Δ/Δ mice. The role of eosinophils in acute or chronic asthma and their role in maintenance of chronic inflammation and airway remodeling is currently a subject of debate. Nevertheless, a large body of published evidence suggests a compelling role of eosinophils in chronic asthma, especially in humans [4]. Although caveats exist in the contribution of eosinophils in different strains of mice [35], our genetic models were of the same genetic background.
The recently appreciated role of anti-stem cell factor (SCF) antibody or oral imatinib mesylate in the attenuation of airway responses in both acute and chronic asthma is intriguing [36]. Interaction of fibroblasts with eosinophils that leads to increased production of cytokines is mediated through SCF [37]. Because of the known influence of SCF on integrin activation [38] and expression of SCF and c-kit in human asthmatic airways [39], it could be speculated that SCF effects are at least partially integrin-dependent, as both c-kit and α4 integrin signaling are linked to the same pathways that regulate migration and activation of mast cells [40]. The role of mast cells, a constant component in allergic inflammatory response in lung, has been controversial in airway disease development [41]. However, abundant experimental evidence in murine models have shown that mast cells adhere to mucosal surfaces through α4β1/ α4β7 and VCAM-1 interactions [42] and secrete important mediators like TNF or CCL1, which can activate Th2 cells, or histamine and leukotriene B4 involved in recruitment of effector T cells in lung [41]. Mice with mast cell deficiency display marked reduction in lung mucosal inflammation, similar to mice with CCR8 deficiency or depletion of CD4 T lymphocytes [43], not unlike our findings with α4Δ/Δ mice. Also consistent with our findings, pretreatments with anti-very late antigen-4 antibody attenuated early response after OVA challenge through inhibition of mast cell activation [44].
New insights about the mast cell-dependent IL-17 activation and its role in asthma have been recently uncovered. IL-17 is thought to be produced by a distinct T cell lineage (Th17 cells), and its production is negatively regulated by IFN-γ and IL-4 [45,46]. After antibody neutralization of IL-17 [47] or in IL-17 knockout mice [48], the OVA-induced initiation of asthma is prevented. Furthermore, mice not susceptible to asthma development (C3H) do not produce IL-17 [49]. These data suggest that IL-17 signaling is essential during antigen sensitization to establish asthma; however, in mice already sensitized, IL-17 seems to attenuate the allergic response [48]. It is of interest that, rather than Th17 cells, the main producers of IL-17 in asthma are alveolar macrophages [47]. Their activation and upregulation of IL-17A is mediated by products secreted by IgE/ OVA-activated mast cells. As the primary sensitization and OVA-specific IgE production in our α4−/− mice is significantly impaired, one may speculate that a decrease in IL-17A is expected in these mice. Although we have not measured levels of IL-17A in acute or chronic asthma in our mice, the ability of naïve CD4+/CD62L+/α4− cells to produce IL-17 is not compromised (Suppl. Fig. 1B and C) under in vitro conditions. Any influence of IL-17, if any, in our mouse model may be secondary to their impaired in vivo activation by mast cells or other cells and lack of migration of effector cells in BALf. Furthermore, induction of Foxp3+/CD103+ regulatory T cells in vitro under the influence of TGF-β is also not impaired in α4−/− T cells (18.3% in α4+/+and 17.8% in α4Δ/Δ).
Among cytokine levels in BALf, there is a striking reduction of TNF-α in α4Δ/Δ mice compared to other groups. Considerable in vitro and in vivo evidence suggests that TNF-α plays a key role in development of AHR, however, the molecular mechanisms linking TNF-α to AHR are not precisely defined [50]. Nevertheless, although we believe that the low levels found in α4Δ/Δ are likely contributing to lack of AHR in these mice, the reasons for the presence of low TNF-α are unclear and require further studies. IL-13 and its downstream target signal transducer and activator of transcription 6 exert a pivotal role in chronic asthma by affecting the function of resident airway cells (e.g., epithelia, fibroblasts, smooth muscle cells, mast cells) and, together with IL-4, by facilitating selective recruitment of inflammatory cells [51,52]. Upregulation of VCAM-1 in airway vessels [14,18] and increase of several chemokines (eotaxin or monocyte chemotactic protein-1, etc.) work in concert to establish chemotactic gradients between different compartments in the lung. In addition, direct participation of IL-13 in fibrosis has been advocated because of IL-13-mediated induction of arginase 1 or TGF-β and platelet-derived growth factor by endothelial cells and monocyte/macrophages and the effects of these mediators on fibroblast proliferation [53,54]. Nevertheless, despite the critical role of IL-13 in chronic asthma [55], IL-13−/− mice still had AHR, albeit with less fibrosis, less inflammatory changes, and intraepithelial eosinophil accumulation, thus dissociating the presence of these changes from AHR [56]. Of interest, IL-13, along with other cytokines, was decreased in BALf of α4Δ/Δ mice, consistent with a decrease in total cells in BALf in these animals, but levels of IL-13 were not different in plasma compared to controls, highlighting the importance of local changes in cytokine/chemokine milieu for asthma development.
A phenotypic response similar to the one seen in our α4Δ/Δ animals in acute and chronic asthma was reported for sphingosine kinase inhibition [57] with suppression of eosinophil migration to airways, Th2 cytokine, and chemokine secretions and decreased AHR. Sphingosine 1-phosphate [57], like α4 integrin, is important in mast cell, neutrophil, and eosinophil chemotaxis, and its inhibition may have an effect on cellular migration machinery involving adhesion molecules.
It is of great interest that, despite the effectiveness of several inhibitors of integrins (i.e., antibodies, small molecules, peptides) in animal models of asthma (i.e., mouse, rat, sheep, guinea pig), human trials have been largely disappointing [23]. It is possible that movement of leukocytes or eosinophils in humans involves other molecules in addition to integrins, or that it involves a combination of integrins (i.e., both β1 and β2 integrins and possibly even some selectins) for dampening the inflammatory response. Furthermore, it should be pointed out that integrin inhibitors largely prevented disease in animals and may not be able to greatly influence already established disease in humans, although they should curtail either the frequency or severity of episodes. Along these lines, our data with the two genetic models (β2−/−,α4Δ/Δ) may provide important preclinical relevance. It could be argued that a major impact of α4 integrin is to dampen the initial sensitization process. As a result, the effectiveness in established processes in humans may be less than that seen in animal models. Further, it is possible that even in the absence of additional inflammatory cell recruitment, the remodeling process persists through sustained activation of airway structural cells [58]. In addition, results we have reported with combined β2 and α4 deficiencies in leukocyte migration to inflammatory sites [59] are instructive in terms of the effectiveness of using combinations of integrin inhibition.
Overall, in chronic asthma there is parallel induction of a complex array of genes in a variety of cells recruited to the airways and in airway resident cells. The contribution of individual players (cells or cytokines) seems to be variable and under independent regulation [60]. We believe that our present studies addressing some of these issues by exploiting genetic murine models expand on the knowledge of the molecular understanding of the pathophysiology of chronic asthma.
Supplementary Material
Acknowledgments
We thank A.L. Beaudet for the β2 deficient mice, G.V. Priestley and D. Batdorf for help with the mice, and G.K.S. Chiang for technical assistance with plethysmography.
This work was supported by National Institute of Health grants HL58734 and DK46557 (T.P.) and AI04289 and HL07322 (W.R.H.). E.R.B., Y.J., and T.P. have no financial relationship with a commercial entity. W.R.H. had the following relationships with commercial interests during the past 12 months: Respiratory Advisory Board at Gilead Sciences, Inc., and lecture fees paid by a commercial entity, i.e., Critical Therapeutics, Inc. and Merck & Co., Inc.
Footnotes
Supplementary data associated with the article can be found in the online version, at doi:10.1016/j.exphem.2009.03.010.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyper-responsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Shum BO, Rolph MS, Sewell WA. Mechanisms in allergic airway inflammation-lessons from studies in the mouse. Expert Rev Mol Med. 2008;10:e15. doi: 10.1017/S1462399408000707. [DOI] [PubMed] [Google Scholar]
- 3.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kariyawasam HH, Robinson DS. The role of eosinophils in airway tissue remodelling in asthma. Curr Opin Immunol. 2007;19:681–686. doi: 10.1016/j.coi.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Benayoun L, Druilhe A, Dombret M-C, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 6.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-d morphometric study. Am Rev Respir Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino M, Nakamura Y, Sim JJ. Expression of growth factors and remodelling of the airway wall in bronchial asthma. Thorax. 1998;53:21–27. doi: 10.1136/thx.53.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:28S–238. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 9.Payne DNR, Rogers AV, Adelroth E, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Yamada G, Saikai T, et al. Increased airway vascularity in newly diagnosed asthma using a high-magnification bronchovideo-scope. Am J Respir Crit Care Med. 2003;168:1495–1499. doi: 10.1164/rccm.200306-727OC. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Li J, Goldsmith AM, et al. Human bronchial smooth muscle cell lines show a hypertrophic phenotype typical of severe asthma. Am J Respir Crit Care Med. 2004;169:703–711. doi: 10.1164/rccm.200307-964OC. [DOI] [PubMed] [Google Scholar]
- 12.Epstein MM. Do mouse models of allergic asthma mimic clinical disease? Int Arch Allergy Immunol. 2004;133:84–100. doi: 10.1159/000076131. [DOI] [PubMed] [Google Scholar]
- 13.Borchers MT, Crosby J, Farmer S, et al. Blockade of CD49d inhibits allergic airway pathologies independent of effects on leukocyte recruitment. Am J Physiol Lung Cell Mol Physiol. 2001;280:L813–L821. doi: 10.1152/ajplung.2001.280.4.L813. [DOI] [PubMed] [Google Scholar]
- 14.Chin JE, Hatfield CA, Winterrowd GE, et al. Airway recruitment of leukocytes in mice is dependent on alpha4-integrins and vascular cell adhesion molecule-1. Am J Physiol Lung Cell Mol Physiol. 1997;272:L219–L229. doi: 10.1152/ajplung.1997.272.2.L219. [DOI] [PubMed] [Google Scholar]
- 15.Henderson WR, Jr., Chi EY, Albert RK, et al. Blockade of CD49d (alpha 4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–3092. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo GC, Shah K, Ding GJF, et al. A small molecule very late antigen-4 antagonist can inhibit ovalbumin-induced lung inflammation. Am J Respir Crit Care Med. 2003;167:1400–1409. doi: 10.1164/rccm.200207-696OC. [DOI] [PubMed] [Google Scholar]
- 17.Laberge S, Rabb H, Issekutz T, Martin J. Role of VLA-4 and LFA-1 in allergen-induced airway hyperresponsiveness and lung inflammation in the rat. Am J Respir Crit Care Med. 1995;151:822–829. doi: 10.1164/ajrccm.151.3.7881677. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S-H, Prince JE, Rais M, et al. Differential requirement for CD18 in T-helper effector homing. Nat Med. 2003;9:1281–1286. doi: 10.1038/nm932. [DOI] [PubMed] [Google Scholar]
- 20.Kanwar S, Smith C, Shardonofsky F, Burns A. The role of MAC-1 (CD11b/CD18) in antigen-induced airway eosinophilia in mice. Am J Respir Cell Mol Biol. 2001;25:170–177. doi: 10.1165/ajrcmb.25.2.4295. [DOI] [PubMed] [Google Scholar]
- 21.Schneider T, Issekutz TB, Issekutz AC. The role of alpha 4 (CD49d) and beta 2 (CD18) integrins in eosinophil and neutrophil migration to allergic lung inflammation in the brown Norway rat. Am J Respir Cell Mol Biol. 1999;20:448–457. doi: 10.1165/ajrcmb.20.3.3207. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee ER, Jiang Y, Henderson WR, Jr., Scott LM, Papayannopoulou T. Alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol. 2007;35:605–617. doi: 10.1016/j.exphem.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 23.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of in-tegrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol. 2008;83:1–12. doi: 10.1189/jlb.0607344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–9360. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwata A, Nishio K, Winn RK, Chi EY, Henderson WR, Jr., Harlan JM. A broad-spectrum caspase inhibitor attenuates allergic airway inflammation in murine asthma model. J Immunol. 2003;170:3386–3391. doi: 10.4049/jimmunol.170.6.3386. [DOI] [PubMed] [Google Scholar]
- 26.Henderson WR, Jr., Chi EY, Maliszewski CR. Soluble IL-4 receptor inhibits airway inflammation following allergen challenge in a mouse model of asthma. J Immunol. 2000;164:1086–1095. doi: 10.4049/jimmunol.164.2.1086. [DOI] [PubMed] [Google Scholar]
- 27.Henderson WR, Jr., Lewis DB, Albert RK, et al. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho JY, Miller M, Baek KJ, et al. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson W, Jr., Banerjee E, Chi E. Differential effects of (s)- and (r)-enantiomers of albuterol in a mouse asthma model. J Allergy Clin Immunol. 2005;116:332–340. doi: 10.1016/j.jaci.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd C, Gutierrez-Ramos J. Animal models to study chemokine receptor function: in vivo mouse models of allergic airway inflammation. Methods Mol Biol. 2004;239:199–210. doi: 10.1385/1-59259-435-2:199. [DOI] [PubMed] [Google Scholar]
- 31.Larbi KY, Allen AR, Tam FWK, et al. VCAM-1 has a tissue-specific role in mediating interleukin-4-induced eosinophil accumulation in rat models: evidence for a dissociation between endothelial-cell VCAM-1 expression and a functional role in eosinophil migration. Blood. 2000;96:3601–3609. [PubMed] [Google Scholar]
- 32.Lobb R, Hemler M. The pathophysiologic role of alpha 4 integrins in vivo. J Clin Invest. 1994;94:1722–1728. doi: 10.1172/JCI117519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee ER, Latchman YE, Jiang Y, Priestley GV, Papayannopoulou T. Distinct changes in adult lymphopoiesis in Rag2(−/−) mice fully reconstituted by alpha4-deficient adult bone marrow cells. Exp Hematol. 2008;36:1004–1013. doi: 10.1016/j.exphem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir LM, Burgess JK, Black JL. Transforming growth factor beta 1 increases fibronectin deposition through integrin receptor alpha 5 beta 1 on human airway smooth muscle. J Allergy Clin Immunol. 2008;121:1034–1039. doi: 10.1016/j.jaci.2007.12.1159. e1034. [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Haczku A, Lee J, Irvin C, Gelfand E. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;281:L394–L402. doi: 10.1152/ajplung.2001.281.2.L394. [DOI] [PubMed] [Google Scholar]
- 36.Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest. 2006;86:557–565. doi: 10.1038/labinvest.3700419. [DOI] [PubMed] [Google Scholar]
- 37.Dolgachev V, Berlin AA, Lukacs NW. Eosinophil activation of fibro-blasts from chronic allergen-induced disease utilizes stem cell factor for phenotypic changes. Am J Pathol. 2008;172:68–76. doi: 10.2353/ajpath.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 39.Al-Muhsen SZ, Shablovsky G, Olivenstein R, Mazer B, Hamid Q. The expression of stem cell factor and c-kit receptor in human asthmatic airways. Clin Exp Allergy. 2004;34:911–916. doi: 10.1111/j.1365-2222.2004.01975.x. [DOI] [PubMed] [Google Scholar]
- 40.Tan BL, Yazicioglu MN, Ingram D, et al. Genetic evidence for convergence of c-kit- and alpha 4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. [DOI] [PubMed] [Google Scholar]
- 41.Reuter S, Taube C. Mast cells and the development of allergic airway disease. J Occup Med Toxicol. 2008;3(Suppl 1):S2. doi: 10.1186/1745-6673-3-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abonia JP, Hallgren J, Jones T, et al. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood. 2006;108:1588–1594. doi: 10.1182/blood-2005-12-012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalo J-A, Qiu Y, Lora JM, et al. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol. 2007;179:1740–1750. doi: 10.4049/jimmunol.179.3.1740. [DOI] [PubMed] [Google Scholar]
- 44.Hojo M, Maghni K, Issekutz TB, Martin JG. Involvement of alpha −4 integrins in allergic airway responses and mast cell degranulation in vivo. Am J Respir Crit Care Med. 1998;158:1127–1133. doi: 10.1164/ajrccm.158.4.9804001. [DOI] [PubMed] [Google Scholar]
- 45.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 46.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song C, Luo L, Lei Z, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 48.Schnyder-Candrian S, Togbe D, Couillin I, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS ONE. 2008;3:e3879. doi: 10.1371/journal.pone.0003879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain D, Keslacy S, Tliba O, et al. Essential role of IFNbeta and CD38 in TNFalpha-induced airway smooth muscle hyper-responsiveness. Immunobiology. 2008;213:499–509. doi: 10.1016/j.imbio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar RK, Herbert C, Webb DC, Li L, Foster PS. Effects of anticy-tokine therapy in a mouse model of chronic asthma. Am J Respir Crit Care Med. 2004;170:1043–1048. doi: 10.1164/rccm.200405-681OC. [DOI] [PubMed] [Google Scholar]
- 53.Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol. 2008;121:560–570. doi: 10.1016/j.jaci.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta (1) J Exp Med. 2001;194:809–822. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nath P, Yee Leung S, Williams AS, et al. Complete inhibition of allergic airway inflammation and remodeling in quadruple IL-4/5/9/13 −/− mice. Clin Exp Allergy. 2007;37:1427–1435. doi: 10.1111/j.1365-2222.2007.02789.x. [DOI] [PubMed] [Google Scholar]
- 56.Kumar RK, Herbert C, Yang M, Koskinen AML, McKenzie ANJ, Foster PS. Role of interleukin-13 in eosinophil accumulation and airway remodelling in a mouse model of chronic asthma. Clin Exp Allergy. 2002;32:1104–1111. doi: 10.1046/j.1365-2222.2002.01420.x. [DOI] [PubMed] [Google Scholar]
- 57.Lai W-Q, Goh HH, Bao Z, Wong WSF, Melendez AJ, Leung BP. The role of sphingosine kinase in a murine model of allergic asthma. J Immunol. 2008;180:4323–4329. doi: 10.4049/jimmunol.180.6.4323. [DOI] [PubMed] [Google Scholar]
- 58.Roth M, Johnson PRA, Borger P, et al. Dysfunctional interaction of C/EBP{alpha} and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med. 2004;351:560–574. doi: 10.1056/NEJMoa021660. [DOI] [PubMed] [Google Scholar]
- 59.Ulyanova T, Priestley G, Banerjee E, Papayannopoulou T. Unique and redundant roles of alpha4 and beta2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Exp Hematol. 2007;35:1256–1265. doi: 10.1016/j.exphem.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koerner-Rettberg C, Doths S, Stroet A, Schwarze J. Reduced lung function in a chronic asthma model is associated with prolonged inflammation, but independent of peribronchial fibrosis. PLoS ONE. 2008;3:e1575. doi: 10.1371/journal.pone.0001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.