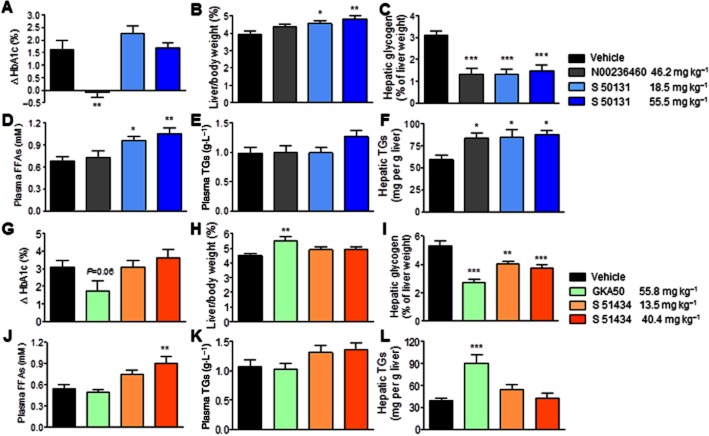
Figure 5.
Evaluation of the efficacy and safety of S 50131 and S 51434 compared to GKA50 and N00236460, after 4 weeks of daily oral administration in db/db mice. The doses expressed as mg·kg−1 correspond to 120 μmol·kg−1 for GKA50 and N00236460, to 60 and 180 μmol·kg−1 for S 50131, and to 1.5-fold lower doses (40 and 120 μmol·kg−1) for S 51434, considering its 1.5-fold greater acute hypoglycaemic potency after 4 h, as shown in Figure 4. (A–F) Efficacy and safety profile of S 50131; (G–L) Efficacy and safety profile of S 51434. (A,G) Differences in plasma HbA1c levels between day 1 and day 28; (B,H) Relative liver weights after 4 weeks; (C,I) Hepatic glycogen content after 4 weeks; (D, J) Plasma free fatty acid (FFA) levels after 4 weeks; (E,K) Plasma triglyceride (TG) levels after 4 weeks; (F,L) Hepatic TG content after 4 weeks. Data are means ± SEM of 12 animals per group. *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from vehicle; anova and Dunnett's multiple comparison test for S 50131 and S 51434; Student's t-test for N00236460 or GKA50.