Scheme 1.
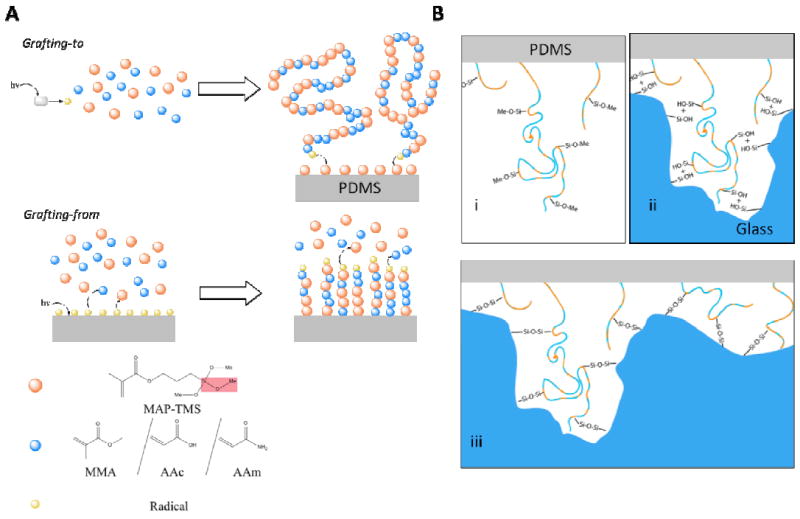
A) Grafting of the polymer is by means of ‘grafting-to’ (top) or ‘grafting-from’ (bottom) reactions. In the former, a photoinitiator (benzophenone, grey rectangle) is activated using a 365 nm UV lamp, generating a radical (yellow) in solution that propagates the polymerization with the dissolved MAP-TMS (orange) or co-polymer, namely methyl methacrylate (MMA), acrylate (AAc), or acrylamide (AAm), (blue). The still-propagating polymer is then grafted onto the MAP-TMS-modified PDMS by reacting with the C=C double bonds on the surface. In ‘grafting-from’ reactions, MAP-TMS-modified PDMS is irradiated with germicidal UV lamp to generate radicals on the elastomer surface. The radicals then propagate a free radical polymerization reaction by first attacking the surface-bound MAP-TMS, followed by the incorporation of the dissolved monomers into the growing polymer chain. The red box in the legend highlights the methoxysilane group, which is the functional unit in bond formation. B) The grafted polymer contains multiple methoxysilane groups (Si-O-Methyl, B. i) which undergo hydrolysis reactions to generate silanols groups (B. ii). These reactive hydroxyls then react with the Si-OH found on glass in a condensation reaction to form covalent Si-O-Si siloxane linkages (B. iii), which is the same reaction in oxygen plasma bonding. The undulation on the glass surface in the figure (B. ii, iii) represents the nanoscale unevenness of the material. The flexibility of our polymer allows it to access the silanols in these nanoscale pits that are inaccessible to non-polymeric silanols in traditional oxygen plasma-treated PDMS (Section 3.2, Supplementary Figure 5). The corresponding unevenness on the PDMS is omitted for clarity.
