Abstract
Purpose
Although studies of serum anti-Müllerian hormone (AMH) in predicting ovarian reserve are numerous, many studies utilized patients under age 40. However, the assessment of ovarian reserve is especially critical in older infertile women. This study evaluates the significance of AMH level in patients over age 40 at the time of their first in vitro fertilization (IVF) treatment.
Methods
Forty-nine women over age 40 were studied. Although serum samples were taken prior to their IVF treatments, the data of serum AMH of patients were not taken into consideration to determine the therapy strategy, including follicle induction in which clomiphene citrate and human menopausal gonadotropin were used.
Result(s)
Twelve out of 49 patients achieved a clinical pregnancy (24.4 %). There was a positive correlation between serum AMH levels and the number of oocytes retrieved (P < 0.0001). The ROC curve analysis for prediction of poor ovarian response, ≤3 retrieved oocytes, showed that the optimum cut-off level was < 1.0 ng/mL for AMH. The lower AMH group (AMH < 1.0 ng/ml) showed less chance of undergoing embryo transfer than the higher AMH group (AMH ≥1.0 ng/ml). There was no difference in pregnancy rate between the two groups. Five out of 12 pregnant women exhibited AMH levels of less than 0.4 ng/ml.
Conclusion(s)
Assessment of serum AMH concentration in older patients is useful for the prediction of oocytes numbers which may be obtained in IVF. A cut-off level of 1.0 ng/ml AMH can be used to predict poor ovarian response. This cut-off level of AMH of 1.0 ng/ml might be useful to predict whether patients could have an embryo transfer, but had no power to predict achieving pregnancy. On the other hand, our data also showed that patients over age 40 with extreme low levels of AMH still had a chance of pregnancy.
Keywords: AMH, Infertility, Aged, IVF
Introduction
In recent years, an increase in higher education and participation in the labor force has resulted in a clear rise in the age at which women deliver their first child [6]. Additionally, there is an evident decline in female fertility with age. The decline in normal fecundity is particularly noticeable over the age of 30, and accelerates between 35 and 40, so that fertility is almost zero by age 45 [12].
For women over age 40, oocyte donation is one solution. However, in several countries where oocyte donation is not permitted, such as Japan, infertility in older women can be difficult to treat.
The decline in female fertility is linked to decreased ovarian reserve [12], thus, proper assessment of ovarian reserve is critical to ensure appropriate treatment. Some patients, classified as “poor responders”, require large doses of stimulation medications and produce suboptimal numbers of mature oocytes. It is essential to identify poor responders prior to entering an IVF program, to aid in management of gonadotropin dosing, and to determine whether effective treatment is possible [2]. To date, basal FSH is the most commonly used test for ovarian reserve estimations. However, the accuracy and clinical value of this test is controversial [1].
Recently, serum anti-Müllerian hormone (AMH) concentration has been used for quantitative evaluation of ovarian reserve [1, 2]. AMH, a member of the transforming growth factor β family, is produced in the granulosa cells [13]. Serum AMH levels have been shown to strongly correlate with the number of antral follicles [10]. Moreover, a stable AMH value is obtainable throughout the menstrual cycle [9]. Several studies have demonstrated that the AMH level is a better marker of ovarian reserve compared with age alone or other markers described in the literature, such as basal FSH, estradiol, and inhibin [2, 9]. AMH level is used most commonly in an IVF setting for the purposes of predicting both over response and poor response in the controlled ovarian stimulation environment [2, 8]. To date, studies of AMH in predicting ovarian reserve have focused on women under age 40. However, the assessment of ovarian reserve is especially critical in older infertile women, where the correlation of AMH levels with ovarian reserve is still unknown. In this study we investigated the significance of AMH in women who underwent their first IVF cycles at the age of 40 and older.
Patients and methods
Forty-nine patients aged 40 or older underwent their first IVF treatment at Denentoshi Ladies Clinic in 2009. On day 3–5 of a spontaneous menstrual cycle, blood samples were obtained by venipuncture before their first IVF. The FSH concentration was measured using the immulite semi-automated assay system. Plasma for assay of AMH was separated immediately from blood and frozen in aliquots at −80 °C until thawed and assayed. The experimental procedures were approved by the institutional review board, and signed informed consent for use of serum was obtained from each patient.
Controlled ovarian stimulation (COS) was carried out by starting clomiphene citrate (Clomid; Shionogi, Tokyo, Japan) at 100 mg per day from days 3 to 7 of the menstrual cycle. Human menopausal gonadotropin (hMG) was given daily from day 3 onward. HMG doses were determined in a flexible manner according to the follicular response monitored with ultrasonography. The gonadotropin-releasing hormone antagonisit Cetrorelix acetate at 0.125 mg was given daily when the leading follicle had reached a size of 16 mm until the day of human chorionic gonadotropin (hCG) injection. When the size of the leading follicle reached 18 mm in mean diameter as measured by transvaginal ultrasound, 5,000 IU of hCG was administered. Oocytes were retrieved transvaginally with ultrasound guidance 36 h after hCG injection. Oocytes were inseminated or injected with husband’s spermatozoa, depending on semen quality. Surplus embryos were frozen and transferred at a later date. Recently, to reduce the risk of multiple conceptions, single embryo transfer is recommended for patients in their first IVF in Japan. Therefore, in the study, single transfer was conducted in all the cases. The luteal phase was supported with progesterone suppositories (400 mg/day) and transdermal estradiol (Estraderm M, Kissei Pharmaceuticals, Japan). After 4 weeks of pregnancy, luteal support with progesterone depot (125 mg per week) was continued up to 7 weeks of gestation, when fetal heartbeat was detected with transvaginal ultrasound.
AMH levels were obtained, using the DSL-10-14400 active Müllerian inhibiting substance/AMH (MIS/AMH) enzyme-linked immunoabsorbent assay (Diagnostic Systems Laboratories, Webster, TX). The minimum detection limit is 0.08 ng/mL. The inter- and intra-assay coefficient of variation is less than 10 %.
In the present study, data of serum AMH of patients were not taken into consideration to determine the therapy strategy, including follicle induction.
Statistical analyses were performed using Statcel (OMS publishing Inc. Japan). The Mann–Whitney U-test, Spearman’s correlation coefficient by rank test and 2 × 2 contingency table analysis were used as appropriate. Significance was defined at a level of P < 0.05.
Results
The mean age and mean FSH level of 49 subjects was 40.9 ± 0.9 years (40–43 years) and 10.6 ± 4.6 IU/L (5.0–27.6 IU/L), respectively. The mean body mass index (BMI) of the subjects was 20.2 ± 2.3 kg/m2 (16.7–28.5 kg/m2). Eight subjects had a history of ovarian cystectomy due to endometriosis. The participants had a variety of causative factors for their subfertility including tubal disease (number, N = 4), male factor infertility (N = 25), combination of tubal disease and male factor (N = 2), and unexplained subfertility (N = 18).
All 49 patients underwent oocyte retrieval. The mean number of oocytes retrieved was 3.9 ± 2.4 (ranged 0–9). In two of the 49 patients, no oocytes were retrieved. After fertilization, 36 of the subjects underwent embryo transfer, and 11 subjects did not have embryos viable for transfer after fertilization. Among 36 patients, 10 patients underwent a frozen embryo transfer without a previous fresh embryo transfer. The reasons of not receiving fresh embryo transfer were as follows: thinning of endometrium (<8 mm at the timing of fresh embryo transfer, N = 3), the presence of endometrial polyp at the timing of fresh embryo transfer, N = 1), serum progesterone > 1.5 ng/ml at the time of hCG injection (N = 2), high serum estradiol at the time of hCG injection as a high risk of ovarian hyperstimulation syndrome (E2 > 2500, N = 2) and other (request by patient, N = 2). Twelve of the patients who underwent embryo transfer achieved clinical pregnancy (fetal heart activity visible on ultrasound scan), giving a conception rate of 24.4 %. Of the 12 patients who achieved a clinical pregnancy, 5 subjects achieved pregnancy as the result of a fresh embryo transfer, 4 subjects achieved pregnancy after a frozen embryo transfer following a previous failure with fresh embryos, and 3 in subjects, pregnancy resulted following a frozen embryo transfer without a previous fresh embryo transfer. Among the 12 clinical pregnancies observed, 9 out of 49 patients (18.3 %) resulted in a live birth.
The median number for serum AMH was 0.58 ng/ml (ranged 0–4.98 ng/ml). Six subjects out of 49 exhibited serum AMH below the detection range.
As mentioned above, eight patients had a history of ovarian cystectomy for endometrial ovarian cysts. The AMH levels of these 8 subjects and the remaining 41 patients were comparable statistically. [P = 0.41, surgical history (+): median 0.46 (0–4.98), surgical history (−): median 0.69 (0–2.89)]. Therefore, the history of surgery was not considered in subsequent analyses.
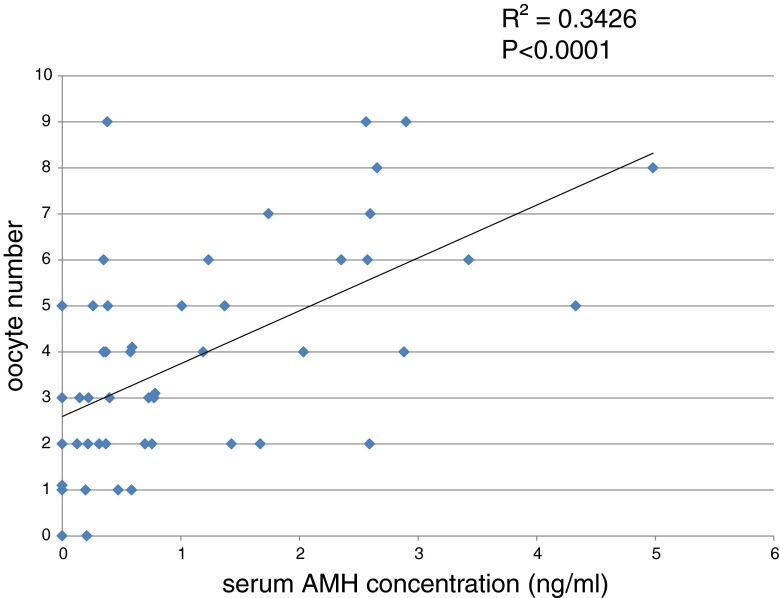
As shown in Fig. 1, there was a positive correlation between serum AMH and the number of oocytes retrieved. (R2 = 0.3324, P < 0.0001). Although serum AMH and FSH exhibited a negative correlation (R2 = 0.06, P < 0.05, data not shown), there was no statically significant correlation between FSH levels and oocyte number (P = 0.095, data not shown).
Fig. 1.
The relationship between serum anti-Müllerian hormone (AMH) concentration and the number of oocytes retrieved during in vitro fertilization treatment (N = 49)
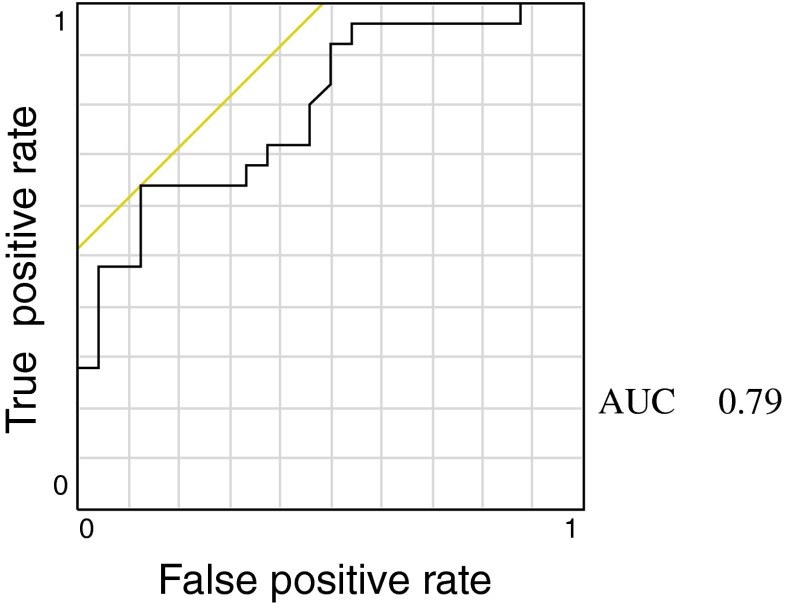
Recently, the definition for poor ovarian response includes the finding that ≤3 oocytes are retrieved with a conventional stimulation protocol [3]. The ROC curve analysis for prediction of poor ovarian response, ≤3 retrieved oocytes, showed that AMH (Fig. 2, ROCAUC = 0.79, P < 0.01) was greater than FSH (ROCAUC = 0.52, P = 0.23). Furthermore, the optimum cut-off level, as indicated by the highest sum of sensitivity and specificity, was ≤ 1.0 ng/mL for AMH. The sensitivity and specificity were 0.875 and 0.64, respectively. When data were re-evaluated without 8 patients who had a history of ovarian cystectomy for endometrial ovarian cysts, AMH cut-off levels of poor responder were also 1.0 ng/ml.
Fig. 2.
Receiver operating characteristic (ROC)-curve for anti-Müllerian hormone (AMH) as an indicator for poor ovarian response (oocyte numbers ≦3) AUC area under the curve
The relationship between serum AMH levels and total dose of gonadotropins during follicle stimulation was examined. In the present study, the physician determined the dose of gonadotropins according to the findings of ultra-sonography and the patient’s serum AMH level was not part of the assessment. A negative correlation between serum AMH levels and total dose of gonadotropins was demonstrated (P < 0.02, data not shown). On the other hand, there was no correlation between the number of oocytes retrieved and the total dose of gonadotropins (P = 0.81, data not shown).
When patients were divided into two groups with cut-off level of AMH of 1.0 ng/ml, 30 and 19 patients were categorized as low and high group, respectively. In the low group, 19 out of 30 patients could undergo embryo transfer (63.3 %), while 17 out of 19 patients could undergo embryo transfer in the high group (89.4 %, P < 0.05). As for pregnancy rate, 5 out of 30 patients in the low group (16.7 %) and 7 out of 19 patients in the high group (36.8 %) achieved pregnancy (P = 0.1, statistically not significant).
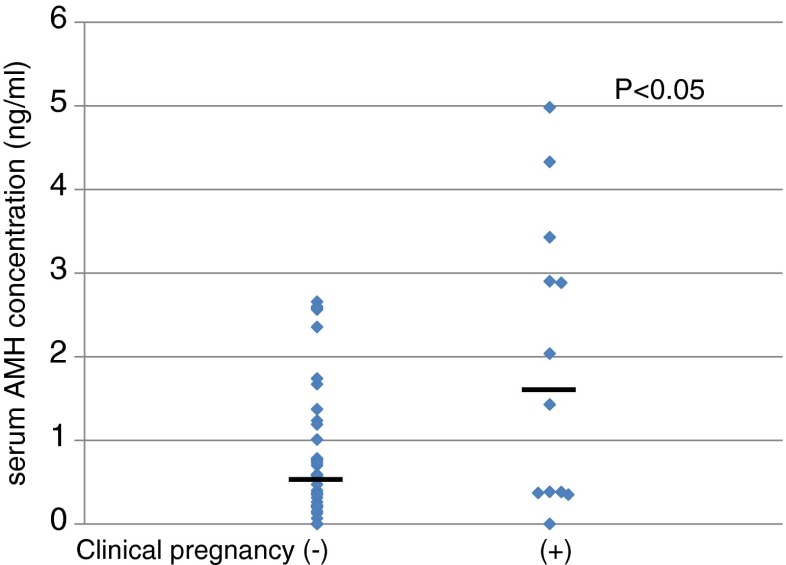
When comparing AMH levels between the pregnant (N = 12) and non-pregnant (N = 37) groups, the pregnant group exhibited significantly higher AMH levels (mean 1.73, range 0–4.98) than the non-pregnant group (mean 0.58, range 0–2.65) (Fig. 3, Mann–Whitney test, P < 0.05), but among the 12 pregnant subjects, 5 exhibited serum AMH levels of less than 0.4 ng/ml, which is considered to be extremely low [14].
Fig. 3.
The distribution of serum anti-Müllerian hormone (AMH) concentration in the non-pregnancy group (clinical pregnancy −, N = 37) and pregnancy groups (clinical pregnancy +, N = 12)
Discussion
It is reported that female age predicts over 80 % of IVF success [11]. Additionally, in women aged 40 and older, ovarian reserve was the main prognostic factor of IVF success [4]. Therefore, for older infertility patients, proper assessment of ovarian reserve, including correct identification of poor responders, is essential. However, until recently a consensus regarding the definition of “poor response” had not been reached. In 2010, the European Society of Human Reproduction and Embryology (ESHRE) set criteria for the definition of poor ovarian response (POR) in IVF. According to the Bologna criteria [3], at least two of the following three features must be present: (i) Advanced maternal age (≥40 years) or any other risk factor for POR; (ii) A previous POR (≤3 oocytes with a conventional stimulation protocol); (iii) An abnormal ovarian reserve test result. Importantly, the criteria designated a serum AMH level of less than 0.5–1.1 ng/ml as an abnormal ovarian reserve test result [3]. Until now, these criteria have only been used to assess patients after completion of IVF treatment, and it remained to be elucidated whether these criteria could be useful to patients prior to initiation of IVF treatment. Moreover, the criteria have been established with data primarily from patients that were less than 40 years old. In this paper, we studied patients who undergo their first IVF cycles at the age of 40 and older, and investigated the significance of AMH levels in these women. Although the blood samples were taken before their first IVF cycle, the AMH data were not taken into consideration in determining gonadotropin dose.
In the present study, there was a positive correlation between serum AMH and the number of oocytes retrieved. (Fig. 1, R2 = 0.3324, P < 0.0001). The ROC curve analysis for prediction of poor ovarian response, ≤3 retrieved oocytes, showed the optimum cut-off level of AMH was < 1.0 ng/mL, which is consistent with the ESHRE criteria indicating a serum AMH level of less than 0.5–1.1 ng/ml as an abnormal ovarian reserve test result [3]. Therefore, among the patients aged 40 and older at the time of their first IVF cycle, the ESHRE criteria proved their validity.
Interestingly, there was a negative correlation between serum AMH levels and total dose of gonadotropins (P < 0.02). On the other hand, there was no correlation between the number of oocytes retrieved and total dose of gonadotropins. These data suggest that the patients who exhibited lower AMH levels would need higher doses of gonadotropins. Therefore, patients with lower levels of serum AMH required additional stimulation to induce follicular development.
When patients were divided into two groups, low and high group, according to cut-off level of AMH of 1.0 ng/ml, the low group had less chance of undergoing embryo transfer (63.3 %) than the high group (89.4 %, P < 0.05). But as for pregnancy rate, 5 out of 30 patients in the low group (16.7 %) and 7 out of 19 patients in the high group (36.8 %) achieved pregnancy. There was a trend, but no statistically significance between two groups (P = 0.1). When comparing AMH levels between the pregnant (N = 12) and non-pregnant (N = 37) groups, the pregnant group exhibited significantly higher AMH levels than the non-pregnant group (Fig. 3). In accordance with higher levels of AMH, the number of oocytes retrieved was significantly greater in the pregnant group (mean 5.4 range 2–9) than in the non-pregnant group (mean 3.4 range 0–9) (P < 0.02, data not shown). Therefore, the more retrieved oocyte number would lead to a higher number of embryos to transfer, thus, increasing the chance of pregnancy. But lower levels of AMH did not indicate that there was no chance of pregnancy. In fact, among the 12 pregnant cases, 5 exhibited serum AMH levels of less than 0.4 ng/ml, which are classified as extremely low levels [14]. Collectively, the levels of AMH may indicate the quantity, i.e., the number of oocytes and embryos, but not the quality of them [5,7], and extremely low AMH levels do not seem to represent an appropriate marker for withholding fertility treatment.
In summary, the present study suggested that serum AMH concentration in older patients might be helpful for the prediction of oocyte numbers which would be obtained in IVF. Moreover, the cut-off level of AMH was 1.0 ng/ml to obtain more than 4 oocytes. This cut-off level of AMH of 1.0 ng/ml might be useful to predict whether patients have a chance for embryo transfer, but had no power to predict achieving pregnancy. On the other hand, our data showed that patients over age 40 with extreme low levels of AMH still had a chance of pregnancy.
Acknowledgments
We thank Ms. Mitsuyo Kakimoto for her editorial assistance. We also thank Dr. Heather M. Martinez for her helpful discussion and critical reading of the manuscript.
Grants
This work was supported by Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan, Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Capsule
In IVF patients over age 40, a cut-off level of 1.0 ng/ml AMH can be used to predict poor ovarian response. But patients with extreme low levels of AMH (<0.4 ng/ml) still had a chance of pregnancy.
References
- 1.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Updat. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 2.Broer SL, Mol BWJ, Hendriks D, Broekmans FJM. Role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705–714. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Ferraretti AP, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Human Reprod (Oxford, England) 2011;26(7):1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto A, et al. Predictive factors of successful pregnancy after assisted reproductive technology in women aged 40 years and older. Reprod Med Biol. 2009;8(4):145–149. doi: 10.1007/s12522-009-0023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khader A, et al. External validation of anti-Müllerian hormone based prediction of live birth in assisted conception. J Ovarian Res. 2013;6(1):3. doi: 10.1186/1757-2215-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leridon H. Demographic effects of the introduction of steroid contraception in developed countries. Hum Reprod Updat. 2006;12(5):603–616. doi: 10.1093/humupd/dml025. [DOI] [PubMed] [Google Scholar]
- 7.Lie Fong S, et al. Anti-Müllerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online. 2008;16(5):664–670. doi: 10.1016/S1472-6483(10)60480-4. [DOI] [PubMed] [Google Scholar]
- 8.La Marca A, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Updat. 2010;16(2):113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 9.La Marca A, Stabile G, Carducci Artenisio A, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Human Reprod (Oxford, England) 2006;21(12):3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 10.Van Rooij IAJ, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Human Reprod (Oxford, England) 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 11.Spandorfer SD, et al. An analysis of the effect of age on implantation rates. J Assist Reprod Genet. 2000;17(6):303–306. doi: 10.1023/A:1009422725434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spira A. The decline of fecundity with age. Maturitas Suppl. 1988;1:15–22. doi: 10.1016/0378-5122(88)90004-7. [DOI] [PubMed] [Google Scholar]
- 13.Weenen C, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 14.Weghofer A, Dietrich W, Barad DH, Gleicher N. Live birth chances in women with extremely low-serum anti-Mullerian hormone levels. Human Reprod (Oxford, England) 2011;26(7):1905–1909. doi: 10.1093/humrep/der134. [DOI] [PubMed] [Google Scholar]