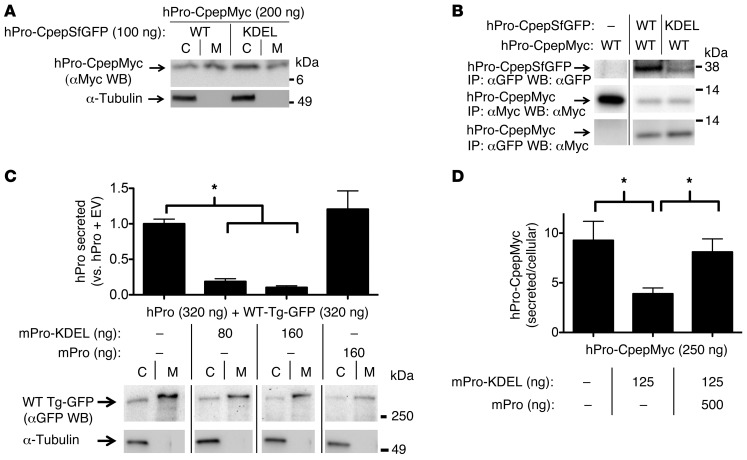
Figure 1. Proinsulin-KDEL interacts with and inhibits secretion of WT proinsulin.
293T cells transiently transfected with hPro-CpepMyc were cotransfected with plasmids as indicated. (A) Cell lysates (C) and media (M) were resolved by SDS-PAGE, electrotransfer, and immunoblotting (WB) with anti-Myc. The media/cell ratio of hPro-CpepMyc bands was decreased by 58.9% ± 12.8% (P = 0.003, n = 6) in cells coexpressing hPro-CpepSfGFP-KDEL compared with that in cells coexpressing WT hPro-CpepSfGFP. (B) Cells lysed in TX-CoIP buffer were immunoprecipitated with anti-GFP or anti-Myc and resolved by SDS-PAGE, electrotransfer, and immunoblotting with anti-GFP or anti-Myc as indicated. The top 2 rows demonstrate expression of the indicated proteins, and the bottom row demonstrates CoIP. Gels are representative of 3 independent experiments. (C) Cells transiently expressing WT hPro plus TgGFP were cotransfected with plasmids as indicated. The media collected overnight were analyzed by hPro-specific RIA. TgGFP in the same cell lysates and media was analyzed by SDS-PAGE, electrotransfer, and immunoblotting with anti-GFP. The media/cell ratio of TgGFP bands in cells coexpressing mPro-KDEL exhibited no significant change compared with that from cells coexpressing WT mPro (1.9 ± 0.2 vs. 2.1 ± 0.8; P = 0.3, n = 5). In B and C, noncontiguous lanes from the same gel are shown. (D) Cells transiently expressing hPro-CpepMyc were cotransfected with plasmids expressing mPro-KDEL or mPro. Media were collected overnight, and cell lysates were analyzed by hPro-specific RIA. The data in C and D represent mean ± SEM, each from ≥4 independent transfections. *P < 0.05.