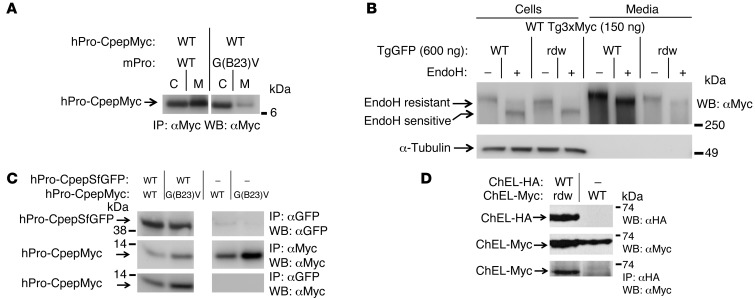
Figure 2. Cross-dimerization of mutant/WT proinsulin and mutant/WT Tg.
293T cells were transiently cotransfected with plasmids expressing the indicated proinsulin or Tg variants. (A) At 48 hours after transfection, cell lysates and overnight media were collected; both were immunoprecipitated with anti-Myc (to prepurify the antigen) and then analyzed by SDS-PAGE, electrotransfer, and immunoblotting with anti-Myc. The media/cell ratio of hPro-CpepMyc bands from cells coexpressing mPro-G(B23)V decreased 66.1% ± 1.9% (P < 0.001, n = 4) compared with that of WT mPro. (B) At 48 hours after transfection, cell lysates and overnight media were collected, treated with or without EndoH, and were analyzed by immunoblotting with anti-Myc. The media/cell ratio of WT Tg3xMyc bands in cells coexpressing rdw-TgGFP decreased 74.1% ± 3.3% (P < 0.001, n = 4) compared with that of WT TgGFP. (C) At 48 hours after transfection, cells lysed in TX-CoIP buffer were immunoprecipitated with anti-GFP or anti-Myc and were analyzed by Western blotting with anti-GFP or anti-Myc, as indicated. (D) Cells transiently coexpressing WT secretory ChEL-HA plus secretory mutant rdw-ChEL-Myc or secretory WT ChEL-Myc (9) were cultured in the presence of brefeldin A (5 hours, 5 μg/ml) to allow intracellular coincubation of the coexpressed constructs. Cells were then lysed in NP40-CoIP buffer and analyzed by immunoblotting with or without immunoprecipitation, as indicated. For C and D, the top 2 rows demonstrate expression of protein partners, and the bottom row demonstrates CoIP. Gels in C and D are representative of n ≥ 3 experiments. In A and C, noncontiguous lanes from the same gel are shown.