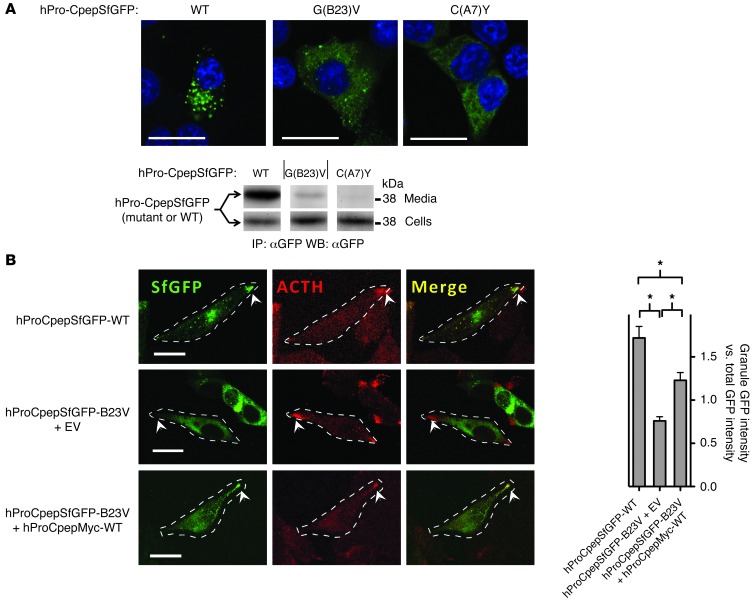
Figure 3. Intracellular distribution of mutant proinsulins in regulated secretory cells coexpressing or not coexpressing WT proinsulin.
(A) Cultured INS1 pancreatic β cells (that express endogenous proinsulin) were transiently transfected to express WT or mutant hPro-CpepSfGFP, as indicated. Fixed cells (counterstained with DAPI) were examined by confocal microscopy for the distribution of SfGFP-containing peptides (scale bar: 20 μm). The cell lysates and overnight bathing media were collected, immunoprecipitated with anti-GFP, and analyzed by immunoblotting with anti-GFP to examine secretion efficiency. Noncontiguous lanes from the same gel are shown. The media/cell ratio for WT, G(B23)V, and C(A7)Y hPro-CpepSfGFP bands was 14.8 ± 3.8, 0.74 ± 0.04, and 0.16 ± 0.06, respectively (P < 0.05 for all groups, n = 4). (B) Cultured AtT20 pituitary corticotroph cells (that do not express endogenous proinsulin) were transiently cotransfected with one of three different plasmid combinations, as indicated. Fixed cells were examined by confocal fluorescence for the distribution of SfGFP-containing peptides (green) and immunofluorescence to localize ACTH-containing secretory granules (red) at the tips of cell (arrowheads; scale bar: 20 μm). Cell boundaries were defined from phase-contrast images (data not shown). Enrichment of average GFP intensity in the secretory granule region was compared with average GFP intensity in nongranule regions. Data represent mean ± SEM from 30 to 38 separately imaged cells for each of the 3 respective transfection conditions. *P < 0.05.