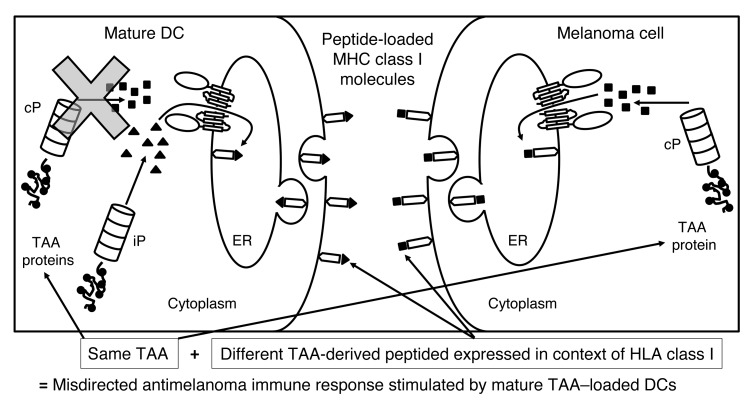
Figure 1. Rationale for the clinical trial.
When TAA-loaded DCs undergo maturation, cPs are no longer expressed; therefore, the TAA-derived peptides presented in the context of HLA class I on the surface of the immunostimulatory mature DCs are exclusively generated by the iP (triangles). CD8+ T cells induced by these DCs will then specifically recognize only iP-generated TAA-derived peptides. In contrast, melanoma cells (as well as other cancer cells) express the cP and not the iP, even when exposed to inflammatory mediators. Therefore, TAA-derived peptides presented in the context of HLA class I on the surface of melanoma cells will be exclusively generated by the cP (squares). T cells induced by the mature TAA–loaded DCs and recognizing iP-generated peptides will then not recognize melanoma cells expressing cP-derived peptides from the same TAA, resulting in a misdirected and suboptimal antimelanoma immune response. By modulating the proteasome of the mature DC from the iP to the cP though transfection of the monocytes from which the DCs were derived with iP siRNA, mature immunostimulatory TAA–loaded DCs then present TAA-derived peptides generated by the cP, stimulating a T cell response that will be appropriately directed against melanoma cells expressing the cP and presenting TAA-derived peptides generated by the cP.