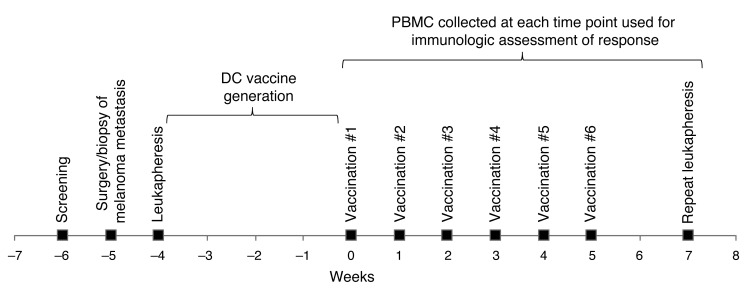
Figure 3. Clinical timeline for vaccine generation and administration and immunologic testing.
Subjects were screened to determine eligibility (week –6), and then underwent surgery or a biopsy of their metastatic melanoma (week –5). Leukapheresis was then performed (week –4) for collection of PBMCs. The monocytes were then isolated from the PBMCs, electroporated with the appropriate siRNAs, and differentiated into immature DCs over a period of 5 days. After induction of maturation for 2 days, DCs were electroporated with TAA RNAs, then cryopreserved. Over the next 3 weeks, the vaccine was test thawed, and both sterility testing and DC phenotypic analysis was performed. Subjects were then vaccinated weekly (weeks 0–5) by intradermal injection, as indicated. Blood was collected at the time of each vaccination, and repeat leukapheresis was performed 2 weeks after the final vaccination (week 7) for isolation of T cells and monocytes, which were used to assess antitumor immune responses.