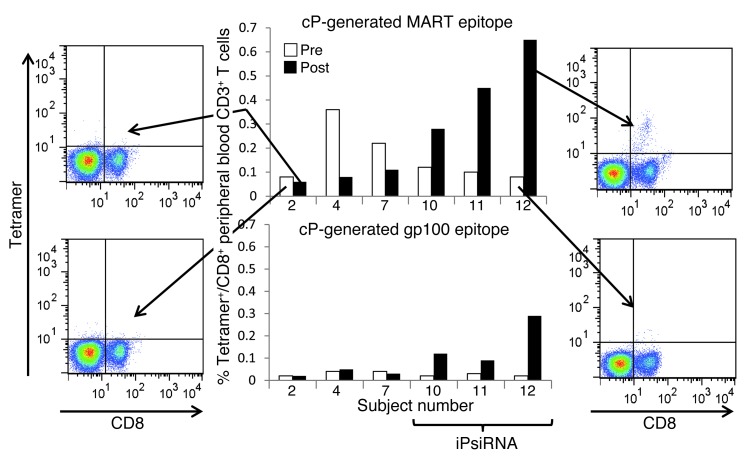
Figure 8. Peripheral blood levels of CD8+ T cells binding HLA-A2 tetramers loaded with melanoma TAA–derived peptides generated by the cP.
For 6 of the 7 subjects in this trial that were HLA-A2+, sufficient numbers of collected peripheral blood T cells were available for tetramer binding analysis. Using blood samples collected before and approximately 1 month after completion of the course of vaccination, PBMCs were isolated and then stained with an anti-CD8 FITC-conjugated mAb and PE-conjugated HLA-A2 tetramers loaded with cP-generated peptides derived from MART1 or gp100. The pre- and postvaccination percentages of CD8+tetramer+ T cells are graphed for subjects 10, 11, and 12 (each vaccinated with TAA RNA–transfected DCs derived from iP siRNA–transfected monocytes), and for subjects 2, 4, and 7, whose TAA RNA–transfected DC vaccine was derived from monocytes that were either untransfected (subjects 2 and 4) or transfected with control siRNA (subject 7). Representative FACS profiles are shown for pre- and postassessment of MART1 CD8+tetramer+ cells for subjects 2 and 12.