FIGURE 13.
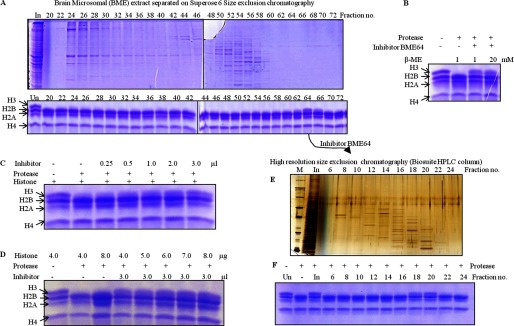
Identification of an inhibitor that inhibits the protease activity of GDH. A, BME was fractionated on the Superose 6 column, and the protein profile was evaluated on 10% SDS-PAGE (upper panel). The presence of an inhibitor specific to GDH was examined by incubating equal volumes of each fraction with core histones and GDH in assay buffer (lower panel) for 2 h at 37 °C. Reaction mixtures were resolved on 15% SDS-PAGE. Undigested brain core histones (Un) were loaded as reference. In, input. The BME fraction 64 was further purified through a high resolution size-exclusion column (Biosuite 125, Waters) (E) and tested for inhibitor activity as above (F). B, increasing concentrations of β-mercaptoethanol did not reverse the effect of inhibitor. Undigested histones and histones with GDH but without inhibitor were incubated as controls. The whole reaction mixture was resolved on 15% SDS-PAGE. C and D, increasing amounts of inhibitor from fraction 64 were added to the assay with proper controls (C). The addition of increasing amounts of core histones to the assay did not reverse inhibition (D). An assay with the highest amount of core histones with GDH and without inhibitor was used as the control (third lane). After incubation for 2 h at 37 °C, reaction products were resolved on 15% SDS-PAGE.
