FIGURE 6.
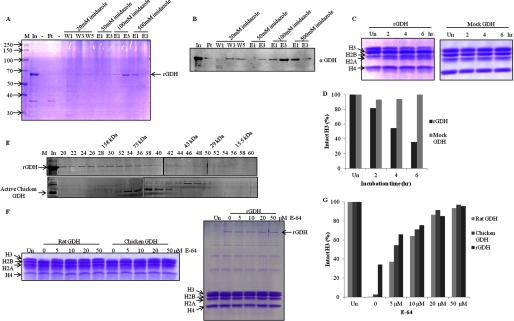
Recombinant mouse GDH shows histone H3-specific protease activity. A, shown is purification of recombinant mouse GDH. The His-tagged mouse GDH was purified from inclusion bodies by urea extraction followed by nickel nitrilotriacetic acid affinity column. M, protein molecular weight marker; In, urea-extracted proteins from inclusion bodies; Ft, flow-through; W1, W2, and W3, first, third, and fifth washes; E1 and E3, first and third elutions at the mentioned imidazole concentrations. The band corresponding to recombinant GDH is marked as rGDH. B, the presence of mouse rGDH through its purification profile was detected by immunoblotting using anti-GDH (anti-GLUD1) antibody of the fractions in A. C, shown is a time point assay of protease activity of rGDH (left panel) and mock GDH (right panel). Un, undigested. D, shown is quantification of protease activity of rGDH compared with mock GDH. E, shown is a comparison of gel filtration profiles of rGDH (upper panel) and chicken liver GDH (lower panel) on Sephacryl S-200 size-exclusion chromatography column. Fractions (numbers on top of the gel) were resolved on 10% SDS-PAGE. M, marker; In, input. Elution positions of molecular weight standards are shown on top. F, shown is an inhibition assay with E-64. The protease activity of GDH purified from rat and chicken liver microsomes was shown to be inhibited by increasing concentrations of E-64 (0, 5, 10, 20, 50 μm) (left panel). E-64 also inhibited protease activity of recombinant mouse GDH (right panel). G, shown is quantification of the protease activity of GDH in the presence of the inhibitor.
