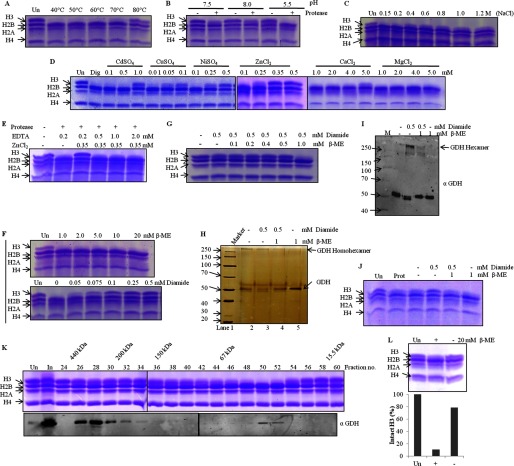
FIGURE 7.
Activity of GDH is regulated by divalent ions, temperature, pH, salt, and thiol-disulfide conversion. Lanes marked Un have undigested histones as references, and M stands for protein marker/ladder. Dig, digestion of H3 upon incubation of core histones with the protease under optimal in vitro assay conditions. A–J, GDH activity on brain core histones was assayed after GDH preincubation for 2 h at various temperatures (A), with buffers at various pH values (B) or supplemented with NaCl (C), divalent cation salts (D), ZnCl2 in the presence of EDTA (E), β-mercaptoethanol (F, top panel), diamide (F, bottom panel), or β-mercaptoethanol with diamide (G). The effect of diamide and/or β-mercaptoethanol on GDH was analyzed after a 1-h treatment by silver-stained SDS-PAGE (H), by Western blotting with anti-GDH antibody (I), with molecular weight markers, and by determining H3-clipping activity (J). K, shown is the presence of monomeric as well as hexameric GDH in vivo. Chicken liver microsomal extract was separated on Superose 6 column, and the fractions were used for protease assay (upper panel). Elution position of molecular mass standards are shown on top of the gel. The protease activity elution coincided with that of monomeric GDH (and not hexameric GDH) in immunoblot analysis using anti-GDH (anti-GLUD1) antibody (lower panel). L, in vivo inactive hexameric GDH shows clipping activity (upper panel) after incubation with β-mercaptoethanol. The H3-clipping activity of hexameric GDH (with or without incubation with β-mercaptoethanol) was quantified (lower panel).