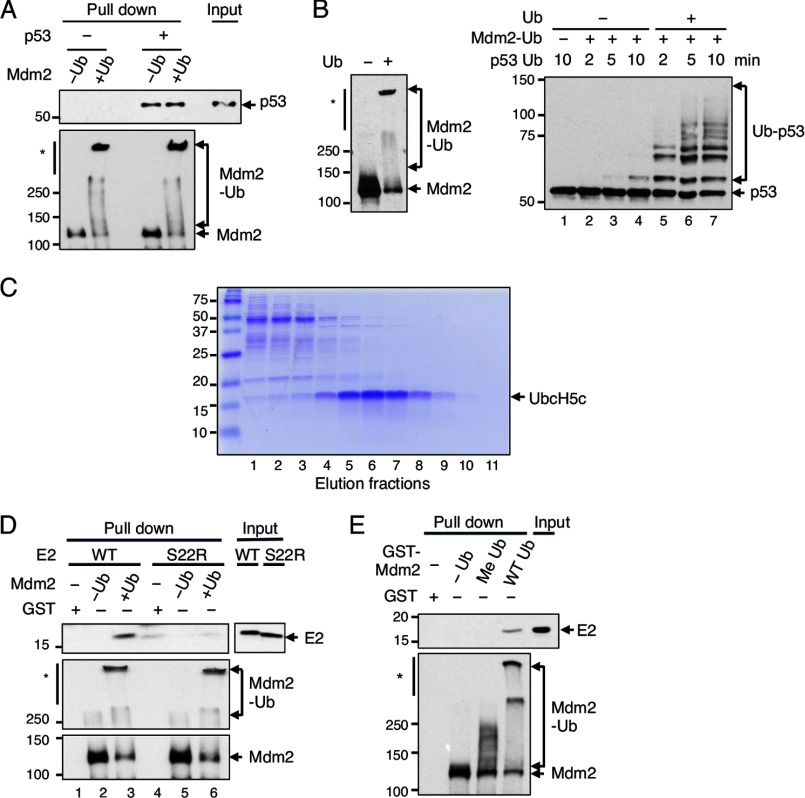
FIGURE 4.
Auto-ubiquitination of Mdm2 does not change affinity for p53 but enhances its recruitment of UbcH5c. A, immobilized GST-Mdm2 with or without auto-ubiquitination incubated alone or with p53. The bound proteins were analyzed by Western blotting with anti-p53 (top) and anti-Mdm2 (bottom) antibodies. The p53 input shown is equivalent to 2.5% of total p53. B, immobilized GST-Mdm2 auto-ubiquitinated (left) and used for p53 ubiquitination without (−) or with ubiquitin (+) in the reaction (right). C, purification of WT UbcH5c. Extracts of BL21 cells expressing UbcH5c were fractionated on a Superdex 200 gel filtration column. Fractions were resolved by SDS-PAGE and stained with Coomassie Blue. The elution profile of S22R UbcH5c was similar. D, in vitro binding of WT UbcH5c or S22R UbcH5c with GST, unmodified GST-Mdm2, or auto-ubiquitinated GST-Mdm2 with minimal reversible cross-linking. Input is 1% of total UbcH5c used for binding. Western blot was analyzed with anti-UbcH5c (top) and anti-Mdm2 (middle and bottom). E, in vitro binding of UbcH5c with GST (lane 1), unmodified GST-Mdm2 (lane 2), mono-ubiquitinated GST-Mdm2 (lane 3), or polyubiquitinated GST-Mdm2 (lane 4) with minimal cross-linking. Input is 0.5% of total UbcH5c used for binding; immunoblotted with anti-UbcH5c (top) and anti-Mdm2 (bottom).