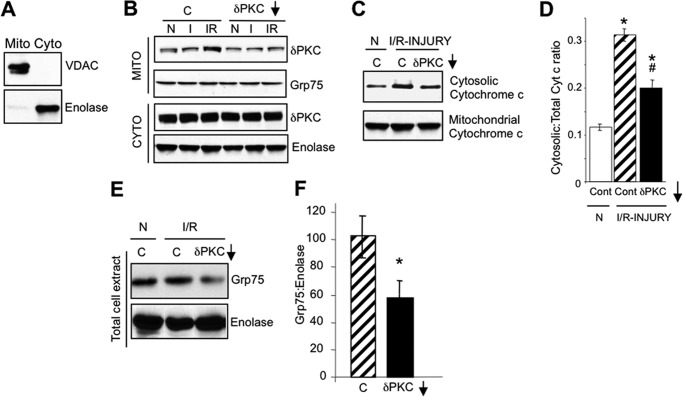
FIGURE 1.
PKCδ translocates to HL1 mitochondria during I/R-induced injury and inhibits mitochondrial elimination. A, Western blots with antibodies to VDAC, a mitochondrial protein, and enolase, a cytosolic protein, showing enrichment of mitochondrial (Mito) and cytosolic (Cyto) fractions from HL1 cells. B, representative PKCδ Western blots of HL1 mitochondrion (Mito)- and cytosol (Cyto)-enriched fractions from HL1 cells subjected to normoxia (N), 2.5 h of ischemia alone (I), or 2.5 h of ischemia followed by 30 min of reoxygenation (I/R) in the absence or presence of a control peptide (C) or the PKCδ translocation inhibitor, δV1–1 (δPKC↓). Blots were stripped and re-probed with anti-Grp75 and anti-enolase as a loading control. C, representative Western blots of anti-cytochrome c in the cytosol- and mitochondrion-enriched fractions from HL1 cells subjected to normoxia or I/R in the presence of a control peptide (C) or the PKCδ translocation inhibitor (δV1–1). D, quantification of Western blots shown in C. Significant differences (p < 0.05) to the normoxic control group (*) and I/R control group (#) were observed. E, representative Western blot showing a reduction of mitochondrial mass (Grp75) in total cell extracts from HL1 cells subjected to normoxia or I/R in the presence of a control peptide (C) or the PKCδ translocation inhibitor, δV1–1 (δPKC↓). The blot was stripped and re-probed with anti-enolase. F, mitochondrial mass following I/R-induced injury was determined by quantifying Western blots described in E, presenting the ratio of Grp75 protein levels to enolase protein levels. Significant differences (*, p < 0.05) to the I/R control group were observed.