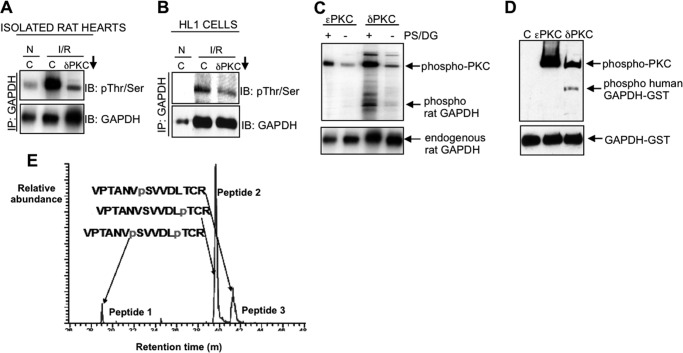
FIGURE 5.
Mitochondrially associated GAPDH is phosphorylated by PKCδ during I/R-induced injury. A and B, isolated rat hearts (A) or HL1 cells (B) were subjected to normoxia (N) or I/R in the presence of the PKCδ translocation inhibitor (δV1–1, indicated by a downward arrow) or control peptide (C). The mitochondrial fraction was immunoprecipitated with a polyclonal antibody to GAPDH and immunoblotted with a mixture of antibodies to phosphorylated serine and threonine, stripped, and re-probed with anti-GAPDH. C and D, in vitro kinase assays containing immunoprecipitated GAPDH from isolated rat hearts (C) or purified recombinant GAPDH-GST alone or in combination with recombinant PKCϵ or recombinant PKCδ (D) were carried out, and the products were immunoblotted with a combination of phosphorylated serine and threonine antibodies (top) or with anti-GAPDH (bottom). PS and DG, cofactors of PKCδ, are abbreviations of phosphatidylserine and diacylglycerol. E, phosphopeptide mapping of the products of the kinase assays described in D. Three phosphorylated peptides were detected in the kinase reaction containing GAPDH-GST and PKCδ only; GAPDH phosphorylated at Ser-241 and Thr-246 (peptide 1), GAPDH phosphorylated at Thr-246 alone (peptide 2), and GAPDH phosphorylated at Ser-241 only (peptide 3). Peptide 2 (Thr-246 alone) comprised over 70% of the entire phosphorylated material detected. No phosphorylation of GST was detected.