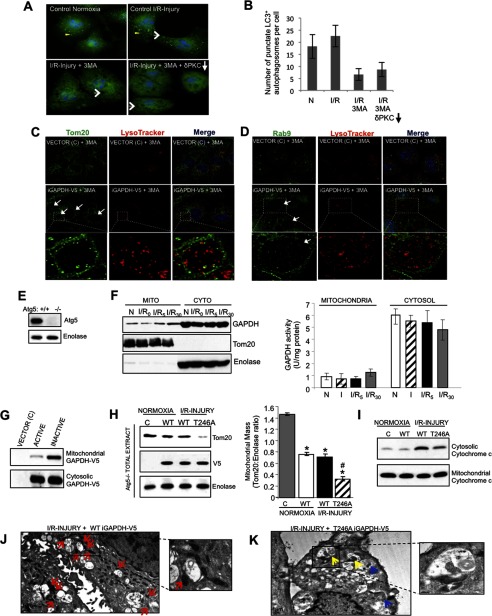
FIGURE 7.
GAPDH-induced mitophagy occurs independently of the macroautophagy pathway. A, HL1 cells maintained under normoxic conditions (left, top panel) or subjected to I/R-induced injury (right, top panel), I/R-induced injury in the presence 100 μm 3MA (left, bottom panel), or I/R-induced injury in the presence of 100 μm 3MA and the PKCδ translocation inhibitor, δV1–1 (right, bottom panel), were fixed and analyzed by immunofluorescence using a rabbit anti-LC3 antibody (green channel). Cells were counterstained with Hoechst (blue channel) to visualize nuclei. Yellow arrowheads indicate LC+ autophagosomes and white arrows indicate the formation of LL structures. Images were acquired at ×100 magnification. B, the number of punctate LC3+ autophagosomes per cell was scored. Results are expressed as the mean ± S.D. (n = 40). C and D, HL1 cells transiently expressing iGAPDH-V5 or control vector (C) were incubated with 3MA for 4 h to inhibit macroautophagy. During the last 30 min, cells were labeled with LysoTracker Red, fixed, and analyzed by immunofluorescence with a deconvolution microscope using anti-Tom20 (A, green channel) or anti-Rab9 (B, green channel) antibodies. LL structures are indicated within white arrows. Images were acquired at ×100 magnification and represent maximum intensity projection of image stacks. The boxed area is expanded in the lower panels and representative LL structures are indicated by white arrows. E, Atg5 Western blot of Atg5+/+ and Atg5−/− MEF cells. F, representative GAPDH Western blot of mitochondrion (Mito)- and cytosol (Cyto)-enriched fractions from Atg5−/− MEFs subjected to normoxia (N) or 2.5 h of ischemia, followed by 0, 5, or 30 min of reoxygenation (I/R). The blot was stripped and re-probed with anti-Tom20 and anti-enolase as indicators of mitochondrial and cytosolic enrichment, respectively. GAPDH activity in mitochondrial and cytosolic fractions was measured (n = 4 per group). There was no significant enzymatic activity of GAPDH in mitochondrial fractions. G, representative Western blot showing that inactive GAPDH (iGAPDH-V5) preferentially associates with mitochondria in Atg5−/− MEFs. Atg5−/− MEFs transiently expressing control vector (C), GAPDH-V5 (active), or iGAPDH-V5 (inactive) under normoxic conditions were enriched for mitochondria and cytosol and analyzed by Western blotting with anti-GAPDH. H, Atg5−/− MEFs transiently expressing control vector (C), iGAPDH-V5, or T246A iGAPDH-V5 were subjected to normoxia or I/R injury. Total cell extracts were analyzed by Western blotting with anti-Tom20. The blot was re-probed for enolase and V5. Quantification of the Western blots is provided. Significant differences (p < 0.05) to the normoxic control group (*) and WT I/R group (#) are indicated. I, Western blotting for cytochrome c levels in the mitochondrion- and cytosol-enriched fractions isolated from mock transfected Atg5−/− MEFs (C) or Atg5−/− MEFs transiently expressing WT iGAPDH-V5 or T246A iGAPDH-V5 and subjected to normoxia or I/R injury. J and K, TEM images of Atg5−/− MEFs transiently expressing WT iGAPDH-V5 (J) and T246A iGAPDH-V5 (K). The cells were subjected to I/R-induced injury. Images were acquired at ×3,000 magnification. Red arrows indicate mitochondria accumulated around LL structures. Yellow arrows indicate perivacuole-associated mitochondria in the process of being directly engulfed, whereas blue arrows indicate mitochondria internalized into LL structures.