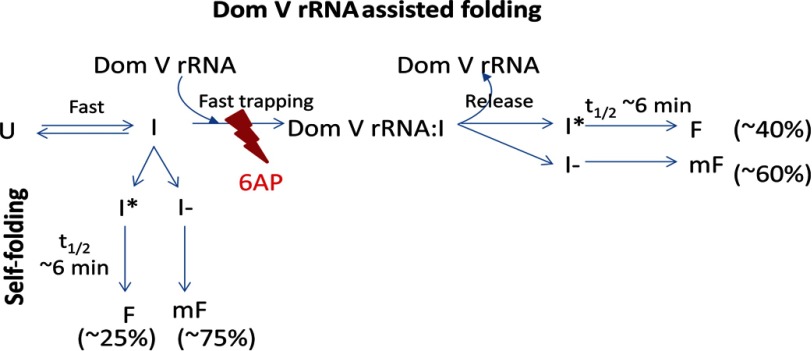
FIGURE 7.
Simple model for PFAR and 6AP action. The unfolded state (U) collapses to an intermediate (I) upon dilution of the denaturant. For self-folding, I transforms either to a folding-competent intermediate (I*), which folds slowly to the native state (F), or to another intermediate (I−), which leads to the misfolded state (mF). The domain V (Dom V) rRNA-assisted refolding proceeds through a fast trapping reaction, which facilitates conversion of I to I*, thereby driving more I molecules to the productive folding pathway (I* → F). 6AP inhibits the trapping reaction by binding to the overlapping sites on domain V rRNA. As a result, the domain V rRNA-assisted pathway gets blocked, and the proteins fold via the self-folding pathway.