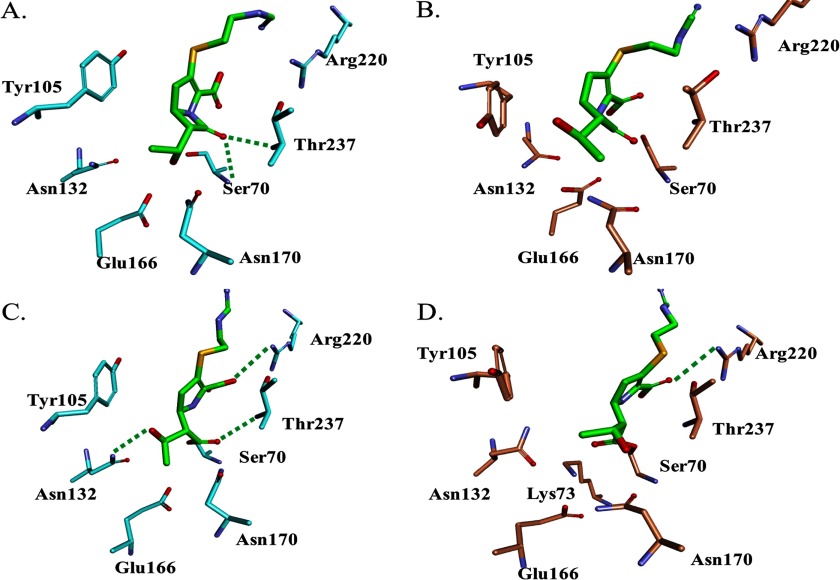
FIGURE 6.
Molecular modeling of PenA and PenI with imipenem. A, Michaelis-Menten complex of PenA (cyan) with IMI (green) shows that the carbonyl of IMI is oriented toward the oxyanion hole, the backbone amides of Ser-70 and Thr-237. B, Michaelis-Menten complex of PenI (orange) with IMI (green) reveals the different conformation of Tyr-105 compared with PenA model. C, Δ2 isoform of IMI (green) with PenA (cyan) reveals the carbonyl of IMI is within the oxyanion hole and in a position favorable for deacylation. D, Δ2 isoform of IMI (green) with PenI (orange) demonstrates that the carbonyl of IMI is outside of oxyanion hole. Water molecules (not shown) are present near Arg-220; thus IMI is in a favorable orientation for tautomerization of its C2–C3 double bond for generation of the more stable Δ1 isoform.