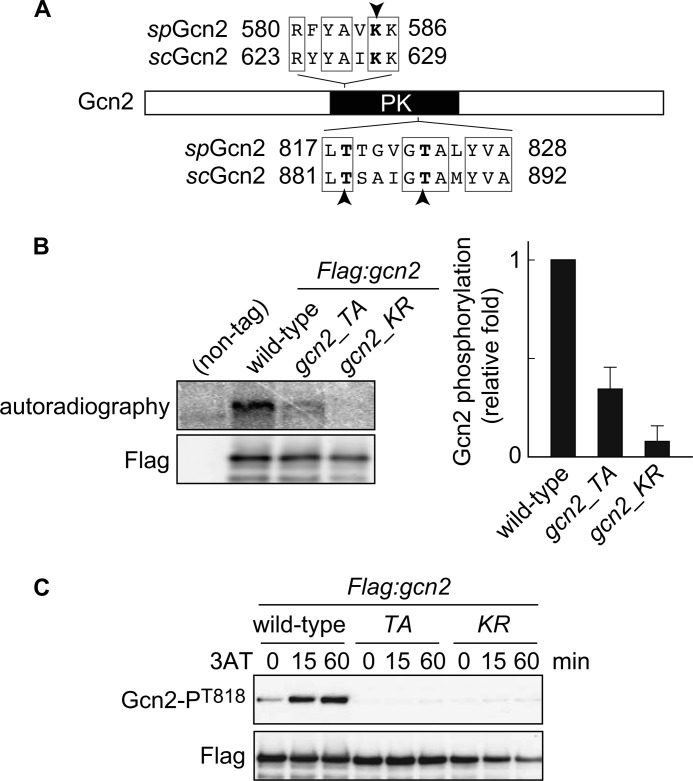
FIGURE 4.
Gcn2 is autophosphorylated in vitro and in vivo. A, domain structure of Gcn2 protein. Lys-628 and threonines Thr-882 and Thr-887, indicated by arrowheads, in S. cerevisiae Gcn2 (scGcn2) are essential sites for kinase activity and autophosphorylation, respectively. The corresponding amino acids in S. pombe Gcn2 (spGcn2) are also shown. PK, protein kinase domain. B, in vitro kinase assay using FLAG-Gcn2 immunoprecipitated from cells harboring FLAG-tagged Gcn2 (Flag:gcn2, YT3372) and nontagged control cells (JK317). Autoradiography and immunoblot probed with anti-FLAG antibody are shown. Flag:gcn2_TA cells (YT3657) have alanine substitution at predicted autophosphorylation sites. Flag:gcn2_KR cells (YT3656) are kinase-dead mutants. Quantified Gcn2 phosphorylation signals (Gcn2P/Gcn2) are shown as the relative fold (S.E. (n = 3)) with respect to the level of Flag:gcn2 cells. C, immunoblots of phosphorylated Gcn2 and FLAG (control) from Flag:gcn2 (YT3372), Flag:gcn2_TA (YT3657), and Flag:gcn2_KR (YT3656) cells treated with 10 mm 3AT. FLAG-Gcn2 was immunoprecipitated before immunoblots.