Abstract
“Snails can kill” is a statement that receives much disbelief. Yet the venom from Conus geographus, as delivered by a disposable hypodermic-like needle, has indeed killed many unsuspecting human victims. Our understanding of their milked venom the essence of these fatalities, is in itself non-existent. Here, we present the molecular mass analysis of the milked venom of C. geographus, providing the first insight into the composition of its deadly cocktail.
Keywords: Cone snail, Toxinology, Fatalities, Conus geographus, Envenomation, Conotoxins, Conopeptides, Mass spectrometry, Milked venom
Twenty-five peptide sequences have been derived from the secretory venom duct of Conus geographus (Table 1; Fig. 1 – insert). This represents a culmination over some ~80 years of biochemical, genetic and pharmacological research.1 Some of these bioactive peptides, commonly called conotoxins or conopeptides, have led to the pharmacological re-classification of ion channels, based on work exploiting isoform selectivity and phyla differentiation characteristics (see Terlau and Olivera, 2004; Table 1). Yet, toxinologically what we know about the composition of these injected venoms remains mostly a mystery, particularly in this species which is known to be lethal to humans.
Table 1.
The conopeptide sequences derived from C. geographus – pharmacological targeting, affinity, origin and expression within the milked venom.
| Name | Amino acid sequence | Target | Affinity IC50 [nM] |
Original source |
Monoisotopic mass (Da) |
Observed m/z in MV |
RP-HPLC Rt (min)a |
Ref.b |
|---|---|---|---|---|---|---|---|---|
| Lys-conopressin G | CFIRNCPKG* | Vasopressin Receptor | N.D. | DV | 1033.49 | 1034.48 | N.D. | [1] |
| α-GII | ECCHPACGKHFSC* | nAChRb | N.D. (LD50 12 μg/kg; mouse) | DV | 1415.5 | 1416.43 | 32.3d | [2] |
| α-GI | ECCNPACGRHYSC* | DV | 1436.48 | 1437.41 | 32.3 | [2] | ||
| nAChR (mouse) | 20 | |||||||
| Site 1 (αδ) | 1.3 ± 0.3 | |||||||
| G5.1 | QGWCCKENIACCV | N.D. | N.D | cDNA | 1451.54 | – | – | [3] |
| α-GIC | GCCSHPACAGNNQHIC* | nAChR (human) | cDNA | 1608.58 | – | – | [4] | |
| hα3β2 | 1.1 | |||||||
| hα3β4 | 775 | |||||||
| hα4β2 | 309 | |||||||
| G1.1 | ECCNPACGRHYSCKG | nAchRb | N.D | cDNA | 1622.58 | 1622.55 | N.D. | [3] |
| α-GIA | ECCNPACGRHYSCGK | nAchRb | N.D | DV | 1622.58 | 1622.55 | 31.0 | [2] |
| Contulakin-G | ZSEEGGSNATKKPYIL | Neurotensin Receptor | DV | 2068.97 | 2068.11 | N.D. | [5] | |
| NTR2 (human) | 960 | |||||||
| NTR3 (mouse) | 250 | |||||||
| α-GID | IRDγCCSNPACRVNNOHVC | nAchR rα7(rat) | 4.5 | DV | 2185.86 | – | – | [6] |
| hα3β2 | 3.1 | |||||||
| hα4β2 | 152 | |||||||
| Conantokin-G | GEγγLQγNQγLIRγKSN* | NMDA Receptor | DV | 2262.94 | – | – | [7, 8] | |
| NR2B | 480 | |||||||
| Brain (human) | 21–69 | |||||||
| μ-GIIIC | RDCCTOOKKCKDRRCKOLKCCA* | NaVc | N.D. | DV | 2595.2 | 2595.02 | 26.4d | [9, 10] |
| μ-GIIIA | RDCCTOOKKCKDRQCKOQRCCA* | NaV1,4 | DV | 2610.14 | 2610.08 | 26.4 | [10, 11, 12] | |
| Brain (rat) | 69.2 ± 0.8 | |||||||
| Brain (chicken) | >1000 | |||||||
| Skeletal muscle (rat) | 0.97 ± 0.17 | |||||||
| Electroplax (eel) | 3.48 ± 0.09 | |||||||
| μ-GIIIB | RDCCTOORKCKDRRCKOMKCCA* | NaV1,4 | DV | 2641.16 | – | – | [10, 13, 14] | |
| NaV1.4 (human) | 1065 | |||||||
| NaV1.4 (rat) | 49 | |||||||
| NaV1.4 (eel) | 1.1 ± 0.1 | |||||||
| ω-GVIC | CKSOGSSCSOTSYNCCRSCNOYTKRC | NaVb | N.D. | DV | 2875.99 | – | – | [10] |
| ω-GVIA | CKSOGSSCSOTSYNCCRSCNOYTKRCY* | CaV 2.2 (chick brain) | 0.15 | DV | 3038.17 | 3038.09 | 32.8 | [10, 15] |
| CaV 2.2 (mouse brain) | 0.07 | |||||||
| ω-GVIB | CKSOGSSCSOTSYNCCRSCNOYTKRCYG | CaVb | N.D. | DV | 3096.13 | 3096.02 | 33.5 | [10] |
| ω-GVIIB | CKSOGTOCSRGMRDCCTSCLSYSNKCRRY | CaVb | N.D. | DV | 3289.34 | – | – | [10] |
| Scratcher peptide | KFLSGGFKγIVCHRYCAKGIAKEFCNCPD* | N.D. | N.D. | DV | 3301.53 | – | – | [15] |
| ω-GVIIA | CKSOGTOCSRGMRDCCTSCLLYSNKCRRY | CaV 2.2 | 3.7 | DV | 3315.39 | 3315.25 | 36.8 | [10, 16] |
| G6.1 | DDECEPPGDFCGFFKIGPPCCSGWCFLWCA | N.D. | N.D. | cDNA | 3322.24 | 3322.13 | N.D. | [3] |
| Conotoxin-GS | ACSGRGSRCOOQCCMGLRCGRGNPQKCIGAHγDV | NaV Skeletal muscle (rat) | 880 | DV | 3617.5 | – | – | [17] |
| Sequence 401 from Patent EP1852440 | SDGGNAAAKESDVIALTVWKCCTIPSC YEKKKIKACVF | N.D. | N.D. | cDNA | 4071.99 | – | – | [18] |
| Sequence 323 from Patent EP1852440 | SDGRNAAANDQASDLMAATVRGCC AVPSCRLRNPDLCGGGR | N.D. | N.D. | cDNA | 4143.86 | – | – | [18] |
| σ-GVIIIA | GCTRTCGGOKCTGTCTCTNSSKCGC RYNVHPSGWGCGCACS* | 5-HT3 | 53 ± 3 | DV | 4189.43 | – | – | [19] |
| Sequence 321 from Patent EP1852440 | SDGRNDAAKAFDLISSTVKKGCCSHPAC AGNNQHICGRRR | N.D. | N.D. | cDNA | 4238.98 | – | – | [18] |
= C-terminal amidation; Z = pyroglutamic acid; S = O-linked glycosylated serine; w = D-tryptophan; W = bromotryptophan; O = 4-trans-hydroxyproline; T = glycosylated threonine; γ = gamma-carboxy glutamic acid; Y = sulfotyrosine.
RP-HPLC retentions are in reference to Fig. 1A.
Corresponding references listed in Supplemental Materials.
Targeting assigned based on sequence homology; N.D., Not Determined, DV, Duct Venom; MV, Milked Venom; cDNA, complementary DNA; nAChR, nicotinic Acetylcholine Receptor; NMDA, N-Methyl-d-aspartate; CaV, Voltage gated calcium channels; NaV Voltage gated sodium channels; 5-HT3, Serotonin 5-HT3-receptor.
Observed as shoulder in primary peak.
Fig. 1.
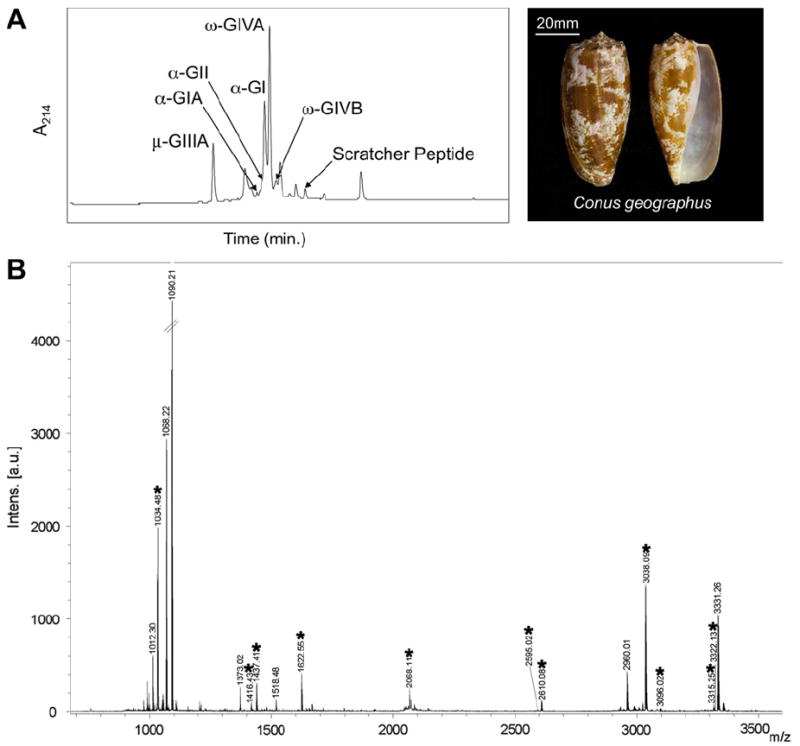
(A) RP-HPLC/UV profile of the milked venom from C. geographus (Insert: C. geographus specimen from Boult Reef, GBR, Australia). (B) The MALDI-TOF-MS analysis of the milked venom from C. geographus. Illustrated is the unexpected molecular mass simplicity of milked venom. Labeled peaks (*) correspond to known conopeptides listed in Table 1. Method: MV was RP-HPLC/UV profiled using a C18 Phenomenex capillary column (5 μm, 300 Å, 1.0 × 250 mm, flow 100 μL min−1) eluted with a linear 1% min−1 gradient of organic [90/10% v/v Acetonitrile (CH3CN)/0.08% v/v TFA aq.] against 0.1% v/v TFA aq. for 80 min, as delivered by a Waters 2695 Alliance HPLC system. Elutant was monitored by photodiode array UV detection from 200 to 300 nm and extracted at 214 nm (A). For MALDI-TOF MS MV analysis, ZipTip® desalted MV was mixed 1:1 with matrix solution (2, 5-dihydroxybenzoic acid [DHB] in 1:1 0.1% v/v TFA aq.: CH3CN) and spotted onto dried matrix saturated in methanol on the MTP 384 polished target plate, and dried under N2. Parent ions were identified on the Ultraflex III MALDITOF-MS (Bruker Daltonics), controlled by the Compass 1.2 SR1 software package in positive reflector mode (B). Peptide II Calibration Mix (Bruker Daltonics) was used for external calibration, with a <5-ppm mass accuracy. Spectra analysis was undertaken using FlexAnalysis v3.0 (Bruker Daltonics).
Potent biological activity has been correlated to the individually isolated secretory duct venom (DV) conopeptides; these have offered some insight into their deadly nature. But there remain a number of compelling issues: Do the complex crude dissected DV extracts represent what the snail uses in prey capture? Are all known DV conopeptides observed within a single milked venom (MV) collection? What makes a ‘killer’ cone snail lethal to humans? Here we address some of these questions using the MV of a known lethal cone snail, C. geographus – this species being responsible for at least 18 human fatalities (see Yoshiba, 1984).
Using a similar method as described by Hopkins et al. (1995), we obtained MV by allowing C. geographus specimens to impale a condom-covered receptacle. The stimulated snails inject venom, under pressure and with velocity, directly into the vial at ~5–20 μL per milking (n = 20). Interestingly, not all C. geographus specimens demonstrated this proboscis extension-envenomation behavior. We collected specimens (n = 4) repeatedly from one specific site, Boult Reef (23°45′32″S 152°16′22″E) in the Capricorn and Bunker Group of Australia’s Great Barrier Reef, which solely demonstrated this unusual predatory behavior. As we have observed, most C. geographus prey envenomations occur within the safety of the rostrum/mouth upon full prey engulfment (n = 22 specimens; 5 locations). The physical environment at Boult Reef may influence C. geographus’ predatory behavior. Specimens are only found in a narrow corridor of fused cavernous coral substrate under large dead plate coral, this provides a Labyrinth forsmall fish. Full expansion of the rostrumto ‘net’ fish would be spatially difficult, making proboscis extension more feasible for predatory success. Observations in captivity show partially opened rostrums during hunting, with a brilliant red proboscis cautiously deployed mid-air a few centimeters ahead, and not as an extended probing appendage as seen in other species. The snail actively pursues the fish; contact between prey and predator is ‘calculated’ targeting the lateral side away from the head and gills; the proboscis is immediately withdrawn upon envenomation leaving the imbedded radula without tethering. The subdued fish, displays a dulled response to physical stimulus and shows labored gill movement, within a few minutes the fish looses the ability to maintain an upright posture. Once on its side, the cone snail moves in with rostrum fully expanded engulfing the fish headfirst, without issue. Milking of C. geographus then becomes a simple intervention once proboscis is visibly extended. Use of whole fish as a milking stimulus increases rate of success.
A representative Reverse Phase-High Performance Liquid Chromatography/Ultra-violet detection (RP-HPLC/UV) profile of non-captive MV (milked within <24 h after field collection) is shown in Fig. 1A. This multi-peak profile, which contains ~12 distinguishable peaks, far contrasts the complexity observed in the crude DV extract (see Olivera et al., 1990; Bingham et al., 1996). A number of C. geographus peptides were isolated and Edman sequenced (not shown), confirming their sequence identity, posttranslational amino acid content and expression – these include, in order of RP-HPLC elution: μ-conotoxin GIIIA (26.4 min), α-conotoxin GI (32.3 min), and ω-conotoxin GVIA (32.8 min). Matrix Assisted Laser Desorption/ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) analysis (positive mode) of the representative single MV sample of C. geographus provided the assignment of 18 molecular masses, of which 12 correlated to known conopeptide sequences (Table 1; Fig. 1B). This was the highest number of known C. geographus conopeptides seen in a single MV from this population. In this example the observed MV mass range encompasses 990–3400, Fig. 1B. The peptide with the highest relative intensity was m/z 1090.21 – an uncharacterized MV constituent. In a number of instances we observed +1 m/z with some of the known conopeptides, as illustrated in Table 1. This potentially represents C-terminal processing, the difference being between the amidated and carboxylated form. This has been a somewhat unexpected observation in this and other Conus MVs (Bingham unpublished results).
Examining the MV molecular mass profile, the dominance of the α-conotoxin family becomes apparent, with 4 individuals identified (α-conotoxins GI, GII, GIA/G1.1; see Table 1). This parallels the common observation of α-neurotoxins in the MV of snakes (Phui Yee et al., 2004). Pharmacologically in C. geographus this is compounded by the co-expression of μ-conotoxins GIIIA, GIIIC (obs. m/z 2610.08, and 2595.02 respectively) and ω-conotoxins GVIA and GVIB (obs. m/z 3038.09, 3096.02 respectively), which completes a predictable peptide toxin ‘motor cabal’ as proposed by Olivera and Cruz (2001). This C. geographus MV ‘cabal’ targets the functionality of pre- and post-synaptic ion channel targets which include: (i) the acetylcholine receptor (α-conotoxins); (ii) the voltage gated sodium channel (muscle type; μ-conotoxins), and; (iii) voltage gated calcium channels (N-type; ω-conotoxins). Here their synergistic pharmacological actions would lead to a rapid and persistent flaccid paralysis – a common observation in both native prey capture (Olivera, 1997) and human envenomations (see Flecker, 1936; Rice and Halstead, 1968).
The expression of Lys-conopression G (calc. m/z 1033.49; obs. m/z 1034.48 – indication of differential C-terminal processing, see above) in the MV raises additional speculation to its biological/pharmacological intention in prey. Suggestions of minimizing prey escape response have been proposed (Dutertre et al., 2008). This has merit as typically C. geographus specimens take to a net-casting-like prey capture response using their rostrum, with the seemingly oblivious fish unaware of the danger it faces while being engulfed alive – a possible indication of prey sedation from material ‘leaking’ from the venom apparatus (see Johnson and Stablum, 1971).
But what of the other conopeptides derived from C. geographus? Or the unidentified m/z observed, as indicated in Fig. 1B? This brings a new level of intrigue and possible explanation of why different outcomes in human envenomation, lethal vs. non-lethal, are reported for this specific species (Cruz and White, 1995). Furthermore, the illustrated MV RP-HPLC (Fig. 1A) may not represent a lethal human dose, as materials recovered are induced by predatory response, are small in relative concentration, and derived from smaller then normal C. geographus specimens – which are typical of Boult Reef. Further investigation is required to see if a ‘defensive milked venom profile’ can be achieved. Our previous observations indicate Conus’ ability to produce ‘dry-milking’s’ and undergo MV differentiation (Chun et al., 2012), which indicates an ability to differentiate and/or control venom secretion.
The presence of additional highly abundant peptides that illustrate unknown or unassigned compounds, i.e. ~40% of the observed MV m/z, provides an indication that even within this well-studied cone snail, C. geographus contains many uncharacterized venom constituents, specifically those observed with a m/z <1100, similar observations are seen in MV from Conus purpurascens (Chun et al., 2012). The primary m/z data provided here may assist in future genomic endeavors using this species and aid in peptide sequence characterization. While with the advancement of low-level peptide detection methods, as too the use of their MVs, as seen here with mass spectrometric incorporation, brings further validation to decades of previous works and now provides direct toxinological insight regarding these lethal predators.
Supplementary Material
Acknowledgments
The authors would like to thank Mr. David Bingham for supplying milked venom, Mr. Jeffrey Milisen for photographic assistance, and the financial support of USDA TSTAR (# 2009-34135-20067) (J-P.B.) and grants from the National Center for Research Resources (5 G12 RR003061-26) and the National Institute on Minority Health and Health Disparities (8 G12 MD007601-26) from the National Institutes of Health.
Abbreviations
- Acetonitrile
CH3CN
- DHB
2, 5-Dihydroxybenzoic acid
- DV
Duct venom
- MALDI-TOF MS
Matrix Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry
- m/z
Mass to charge ratio
- MV
Milked venom
- Obs. m/z
Observed Mass to charge ratio
- RP-HPLC
Reverse Phase-High Performance Liquid Chromatography
- TFA aq.
Tri-fluoroacetic acid/aqueous
- UV
Ultra-violet detection
Footnotes
This paper is dedicated to a mentor, friend and fellow shell collector, Associate Professor Bruce G. Livett, formerly of the Department of Biochemistry & Molecular Biology at the University of Melbourne, Australia, in celebration of his retirement and contribution to the field of conopeptide research.
Much of this research has been reported in Toxicon over the last 50 years.
Appendix A. Supplementary material
Supplementary material associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxicon.2012.07.014.
Conflict of interest
Authors state that there is no conflict of interest.
Ethical statement
The author and co-authors of this paper have acted ethically in conducting the described research, having undertaken careful analysis of data and the submitted manuscript to avoid errors.
References
- Bingham J, Jones A, et al. Conus venom peptides (Conopeptides): inter-species, intra-species and within individual variation revealed by ionspray mass spectrometry. In: Lazarovici P, Spira ME, Zlotkin E, editors. Biochemical Aspects of Marine Pharmacology. Alaken Inc.; Fort Collins, Colorado, USA: 1996. pp. 13–27. [Google Scholar]
- Chun JB, Baker MR, et al. Cone snail milked venom dynamics – a quantitative study of Conus purpurascens. Toxicon. 2012;60(1):83–94. doi: 10.1016/j.toxicon.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz LJ, White J. Clinical toxicology of Conus snail stings. In: Meier J, White J, editors. Clinical Toxicology of Animal Venoms. CRC Press; Boca Raton, FL: 1995. pp. 117–127. [Google Scholar]
- Dutertre S, Croker D, et al. Conopressin-T from Conus tulipa reveals an antagonist switch in vasopressin-like peptides. J Biol Chem. 2008;283(11):7100–7108. doi: 10.1074/jbc.M706477200. [DOI] [PubMed] [Google Scholar]
- Flecker H. Cone shell poisoning, with report of a fatal case. Med J of Aust. 1936;1:464–466. [Google Scholar]
- Hopkins C, Grilley M, et al. A new family of Conus peptides targeted to the nicotinic acetylcholine receptor. J Biol Chem. 1995;270(38):22361–22367. doi: 10.1074/jbc.270.38.22361. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Stablum W. Observations on the feeding behavior of Conus geographus (Gastropoda: Toxoglossa) Pac Sci. 1971;25(1):109–111. [Google Scholar]
- Olivera BM, Cruz LJ. Conotoxins, in retrospect. Toxicon. 2001;39(1):7–14. doi: 10.1016/s0041-0101(00)00157-4. [DOI] [PubMed] [Google Scholar]
- Olivera BM, Rivier J, et al. Diversity of Conus neuropeptides. Science. 1990;249(4966):257–263. doi: 10.1126/science.2165278. [DOI] [PubMed] [Google Scholar]
- Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 1997;8(11):2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phui Yee JS, Nanling G, et al. Snake postsynaptic neurotoxins: gene structure, phylogeny and applications in research and therapy. Biochimie. 2004;86(2):137–149. doi: 10.1016/j.biochi.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rice RD, Halstead BW. Report of fatal cone shell sting by Conus geographus Linnaeus. Toxicon. 1968;5(3):223–224. doi: 10.1016/0041-0101(68)90093-7. [DOI] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84(1):41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Yoshiba S. An estimation of the most dangerous species of cone shell, Conus (Gastridium) geographus Linne, 1758, venom’s lethal dose in humans. Jpn J Hyg. 1984;39(2):565–572. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


