Paleoanthropologists have long relied on skull and tooth morphology to infer fossil hominin diets, but from the early 1980s, they have also looked to microscopic wear traces in dental enamel, and since the early 1990s, they have looked increasingly to the stable isotope composition of skeletal tissues. The most commonly used stable isotopes are varieties of the light elements nitrogen and carbon. Nitrogen isotope ratios can provide information about the degree of carnivory vs. herbivory (1), but nitrogen can be extracted only from fossil bones that retain protein, which means specimens mostly younger than 100,000 y in temperate latitudes and usually much younger than 25,000 y closer to the Equator. In contrast, antemortem carbon isotope ratios persist indefinitely in dental enamel (2), and the main limitation is that they reflect ancient diets mostly in tropical or subtropical settings where the plants divide subequally between ones that follow a C4 photosynthetic pathway and others that follow a C3 pathway. This is not a problem for paleoanthropology, because early hominins (humans broadly understood) evolved in tropical and subtropical Africa, and their teeth are preserved in numerous African sites. Four articles in PNAS show how stable carbon isotopes have illuminated the diets of hominins that lived in Africa between roughly 4.1 and 1.3 Ma.
Link Between Carbon Isotopes and Ancient Diets
In tropical and subtropical environments, the tissues of plants that follow a C4 photosynthetic pathway, mainly grasses and some sedges, tend to be enriched in 13C relative to 12C. In contrast, the tissues of plants that follow a C3 pathway, mainly trees, shrubs, bushes, and forbs, tend to be much poorer in 13C. The tissues of plants that follow the Crassulacean acid metabolism (CAM) photosynthetic pathway, mainly succulents, resemble C4 plants in 13C enrichment, but they were probably rarely important to early hominins, because they occur mostly in deserts. Dietary reconstruction depends on the observation that the 13C/12C ratios in herbivores reflect the ratios in the plants they eat, and the ratios in carnivores reflect the ratios in their prey.
The 13C/12C ratio is recorded in various tissues, but from a fossil perspective, dental enamel is by far the most important. This is because its density promotes survival, and it resists postdepositional chemical alteration (diagenesis). It thus retains the antemortem 13C/12C signal. Enamel that is especially enriched in 13C normally implies a diet composed largely of grasses or animals, including insects, that eat grasses. Enamel that is significantly depleted in 13C implies a diet that included mostly nuts, fruits, leaves, or other parts of nongrassy plants or of animals that eat such plants. Values are usually expressed as a departure in 13C per mil (=δ13C ‰) from an accepted standard, and they have been determined for a variety of African savanna ungulates (3). ∂13C ‰ in the enamel of species that feed on C4 grasses commonly ranges between 1.7 and 3.9. In the enamel of species that feed on nongrassy plants, it typically ranges between −14.9 and −10.2.
Stable Carbon Isotopes in Dental Enamel and Early Hominin Environments
In general, stable carbon isotopes reveal only what a species selected from the surrounding vegetation and not the overall vegetational community. However, if the 13C/12C ratio shifts over long intervals in the teeth of species that were almost certainly grazers, a reasonable inference is that there was a shift in the nature of the available grass. Thus, an increase in 13C enrichment in grazer enamel across multiple lineages implies that C4 grasses spread at the expense of C3 plants in tropical and subtropical latitudes between 8 and 3 Ma (4, 5). This is paleoanthropologically significant, because it supports the “savanna hypothesis,” according to which hominin bipedalism represents an adaptive response to the increasingly patchy distribution of trees or tree stands vs. grasses in tropical and subtropical Africa beginning roughly 8 Ma.
By 2 Ma or so, African savannas closely resembled historic ones and fossil ungulates mainly anticipated living ones such as equids (zebras), alcelaphine (wildebeest/hartebeest) antelopes, reduncine (waterbuck/reedbuck) antelopes, and hippotragine (roan/sable) antelopes that feed primarily on C4 grasses (grazers) and others such as giraffids and tragelaphine (kudu/bushbuck) antelopes that feed primarily on the leaves of C3 bushes, shrubs, and trees (browsers). Enamel 13C/12C ratios show that fossil species after 2 Ma divide between grazers and browsers exactly as their anatomical resemblances to living species would predict (6), which confirms that the grazer/browser ratio in a fossil site can be used to estimate the relative abundance of grass vs. bush nearby, or more conservatively, that differences in grazer/browser ratios between sites can be used to gauge differences in the abundance of grass vs. bush nearby.
Stable Carbon Isotopes in Dental Enamel and the Diet of Early Hominins
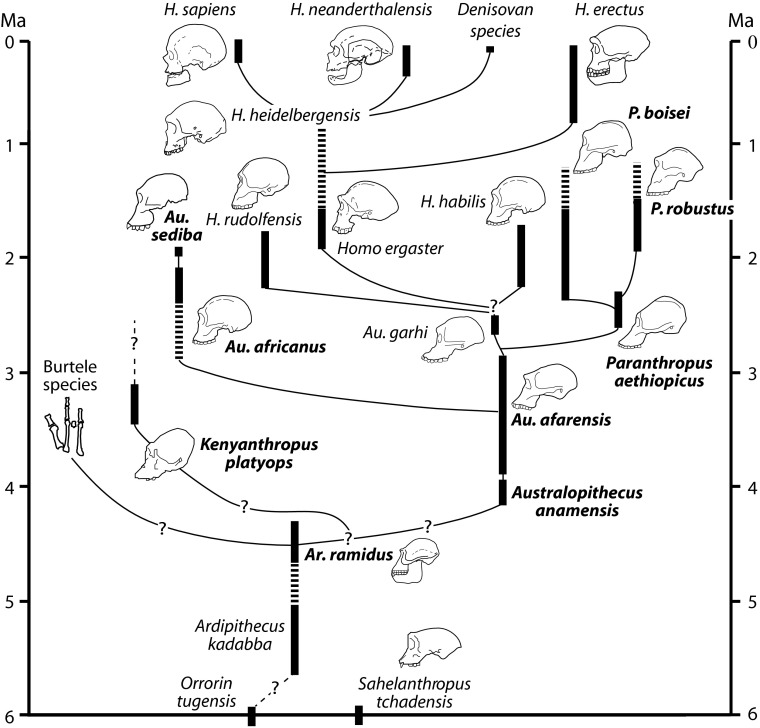
Fig. 1 presents the time spans and putative evolutionary relationships of the hominin species mentioned below.
Fig. 1.
Hypothetical phylogeny of the Hominini. Boldface marks species for which stable carbon isotope observations are available. Observations for early Homo have thus far been presented without reference to species. Broken bars indicate probable as opposed to directly established time spans.
In paleoanthropology, the first application of stable carbon isotope analysis was to South African Australopithecus africanus (∼2.8–2.1 Ma), Paranthropus robustus (∼2–1.2 Ma), and early Homo (∼2 Ma). The results indicated that the dental enamel of each species was relatively enriched in 13C and to about the same extent (7, 8). This has been interpreted to mean that, on average, each species obtained 25–30% of its carbon from grassy (C4) foods even if it obtained the bulk from berries, nuts, fruits. leaves, or other C3 items. In contrast, it was recently shown that the enamel of the east African australopith, Paranthropus boisei (2.3–1.2 Ma), is much richer in 13C, and as stressed below, the degree of enrichment implies that it obtained 75–80% of its diet from C4 sources (9). Carbon isotopes cannot pinpoint the specific grass-based foods that the various species consumed, but significant differences in jaw and tooth morphology and in microwear (7) suggest that the different species exploited different sources. For A. africanus and P. robustus, the principal ones may have been grass seeds, grass roots, the underground storage organs (USOs) of sedges, and termites, whereas for early Homo, the flesh or marrow of grazing ungulates may also have been important. For P. boisei, the principal source may have been especially large quantities of sedge USOs. The great difference in 13C enrichment between P. boisei and P. robustus was unexpected, because the species are remarkably similar in skull and tooth morphology. However, they also differ in their enamel microwear (9), and the implication may be that they did not share an especially close common ancestor but evolved their craniodental similarities in parallel.
In PNAS, Cerling et al. (10) report on stable carbon isotopes in the teeth of hominins that lived between 4.1 and 1.4 Ma in the Lake Turkana basin of northern Kenya. The results indicate that the oldest species, Australopithecus anamensis (4.2–4.0 Ma), rarely consumed C4 foods, even though these were probably readily available nearby. In its strong C3 preference, A. anamensis recalls its putative ancestor, Ardipithecus ramidus (11), and also the common chimpanzee, which favors C3 foods almost exclusively even in relatively open savanna settings where potential C4 foods abound (12). In contrast to the results for A. anamensis, those for Kenyanthropus platyops (3.6–3.0 Ma), which was coeval and possibly conspecific with Ethiopian Australopithecus afarensis (13), sometimes indicate significant consumption of C4 foods. The results for Paranthropus aethiopicus (2.6–2.3 Ma) and for sympatric P. boisei and early Homo (2–1.5 Ma), reveal a persistent interest in C4 foods, but as noted previously, the degree in P. boisei was exceptional. Cerling et al. estimate that it consumed 75% C4 items compared with 35% in early Homo, although Homo increased its C4 consumption toward 1.5 Ma, perhaps because it fed increasingly on the flesh of grazing ungulates.
Wynn et al. (14) report on stable carbon isotopes in the teeth of later Australopithecus afarensis (3.4–2.9 Ma) from localities in the Hadar region of north-central Ethiopia. A. afarensis is generally thought to have descended from A. anamensis about 3.8 Ma, and it is a likely common ancestor for all hominins that postdate 2.6 Ma, including A. africanus, Paranthropus spp., and Homo. Carbon isotope ratios show that later A. afarensis consumed C4 foods to about the same extent as most subsequent hominins, and A. afarensis is thus the oldest species to document the conspicuous dietary divide between hominins and chimpanzees. Among later species, only Australopithecus sediba, dated to about 2 Ma at Malapa Cave, South Africa, may have resembled chimpanzees in a strong preference for C3 foods (15). This may mean it was as peculiar in its diet as it was in its anatomy, but secure dietary inference requires an enlarged sample of analyzed teeth.
Sponheimer et al. (8) review the principles that allow dietary inferences from stable carbon isotopes. They summarize all of the available observations, involving 175 teeth from multiple early hominin species between 4.4 and 1.3 Ma, and they explore the likely relationship between inferred diets and the form of early hominin jaws and teeth. They suggest that the increased interest in C4 foods by australopiths after 4 Ma could largely explain a broadly simultaneous expansion in the chewing surfaces of the postcanine (premolar and molar) teeth and in the thickness of the mandibular body. They also stress that the trend toward increasing C4 consumption after 4 Ma is nearly unique to the hominins. Coeval mammal species remain consistently oriented toward C4, C3, or mixed C3/C4 diets. The trend in hominins is thus likely to reflect fundamental evolutionary change.
Finally, Cerling et al. (16) report on carbon isotope ratios in enamel of the extinct gelada baboons Theropithecus brumpti (4-2.5 Ma) and Theropithecus oswaldi (2.0–0.9 Ma). Fossils of both species frequently accompany those of hominins in eastern Africa, especially in Kenya from which Cerling et al. obtained their samples. T. oswaldi is thought to have descended from Theropithecus darti, a broad contemporary of T. brumpti in southern Africa, and to have replaced T. brumpti in eastern Africa after 2.5–2 Ma. Isotope ratios for all three fossil Theropithecus species imply they favored grassy C4 foods, but this was especially true for T. oswaldi, for which the ratios suggest 80% C4 consumption. The difference from T. brumpti may reflect a difference in surroundings, because associated mammals suggest that T. oswaldi occupied more open savanna settings. In the degree to which extinct geladas favored grassy foods, they anticipate their smaller, surviving relative, Theropithecus gelada, which feeds almost exclusively on grass stems and seeds in the central Ethiopian highlands. Among other known primates, living or extinct, only P. boisei approximated the geladas in its C4 emphasis. Geladas evolved unusually high-crowned postcanine teeth to masticate large quantities of grassy items. P. boisei may have evolved unusually large postcanine tooth surfaces for the same purpose.
Conclusion
Twenty years ago, we might have guessed, based mainly on the savanna settings in which early hominins evolved, that they depended increasingly on grassy foods or on creatures that ate grasses. The craniodental morphology of P. boisei might also have led to us to speculate that it relied on grassy foods to a particularly great extent, rivaling only gelada baboons among known primates. Now, thanks to stable-isotope analyses, we no longer have to guess, and the broad pattern of early hominin dietary evolution is established. Continuing isotope research will tell us what early A. afarensis (3.8–3.4 Ma) is likely to have eaten, whether the diet of A. sediba was truly chimpanzee-like, and perhaps most interesting, whether different diets distinguished the three species of early Homo that are now thought to have coexisted between 2 and 1.5 Ma (17).
Footnotes
References
- 1.Richards MP, Trinkaus E. Out of Africa: Modern human origins special feature: isotopic evidence for the diets of European Neanderthals and early modern humans. Proc Natl Acad Sci USA. 2009;106(38):16034–16039. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee-Thorp JA, van der Merwe NJ, Brain CK. Isotopic evidence for dietary differences between two extinct baboon species from Swartkrans. J Hum Evol. 1989;18(3):183–189. [Google Scholar]
- 3.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120(3):347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 4.Cerling TE, et al. Global vegetation change through the Miocene/Pliocene boundary. Nature. 1997;389(6647):153–158. [Google Scholar]
- 5.Uno KT, et al. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proc Natl Acad Sci USA. 2011;108(16):6509–6514. doi: 10.1073/pnas.1018435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerling TE, Levin NE, Passey BH. Stable isotope ecology in the Omo-Turkana Basin. Evol Anthropol. 2011;20(6):228–237. doi: 10.1002/evan.20326. [DOI] [PubMed] [Google Scholar]
- 7.Grine FE, Sponheimer M, Ungar PS, Lee-Thorp J, Teaford MF. Dental microwear and stable isotopes inform the paleoecology of extinct hominins. Am J Phys Anthropol. 2012;148(2):285–317. doi: 10.1002/ajpa.22086. [DOI] [PubMed] [Google Scholar]
- 8.Sponheimer M, et al. Isotopic evidence of early hominin diets. Proc Natl Acad Sci USA. 2013;110:10513–10518. [Google Scholar]
- 9.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110:10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 12.Sponheimer M, et al. Do “savanna” chimpanzees consume C4 resources? J Hum Evol. 2006;51(2):128–133. doi: 10.1016/j.jhevol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 13.White TD. Paleoanthropology. Early hominids—Diversity or distortion? Science. 2003;299(5615):1994–1997. doi: 10.1126/science.1078294. [DOI] [PubMed] [Google Scholar]
- 14.Wynn JG, et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. Proc Natl Acad Sci USA. 2013;110:10495–10500. doi: 10.1073/pnas.1222559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry AG, et al. The diet of Australopithecus sediba. Nature. 2012;487(7405):90–93. doi: 10.1038/nature11185. [DOI] [PubMed] [Google Scholar]
- 16.Cerling TE, Chritz KL, Jablonski NG, Leakey MG, Manthi FK. Diet of Theropithecus from 4 to 1 Ma in Kenya. Proc Natl Acad Sci USA. 2013;110:10507–10512. doi: 10.1073/pnas.1222571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leakey MG, et al. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature. 2012;488(7410):201–204. doi: 10.1038/nature11322. [DOI] [PubMed] [Google Scholar]