Abstract
Hemoglobins are recognized today as a diverse family of proteins present in all kingdoms of life and performing multiple reactions beyond O2 chemistry. The physiological roles of most hemoglobins remain elusive. Here, we show that a 2-on-2 (“truncated”) hemoglobin, termed THB8, is required for hypoxic growth and the expression of anaerobic genes in Chlamydomonas reinhardtii. THB8 is 1 of 12 2-on-2 hemoglobins in this species. It belongs to a subclass within the 2-on-2 hemoglobin class I family whose members feature a remarkable variety of domain arrangements and lengths. Posttranscriptional silencing of the THB8 gene results in the mis-regulation of several genes and a growth defect under hypoxic conditions. The latter is intensified in the presence of an NO scavenger, which also impairs growth of wild-type cells. As recombinant THB8 furthermore reacts with NO, the results of this study indicate that THB8 is part of an NO-dependent signaling pathway.
Keywords: gene expression, hypoxia
Hemoglobins (Hbs) (globular proteins with a heme prosthetic group) were discovered as O2-transporting molecules in animal blood. Today they are recognized as a diverse family of proteins as genomic data mining has resulted in the discovery of hundreds of new Hbs in all kingdoms of life (1, 2). Hbs feature either the canonical 3-on-3 α-helical so-called myoglobin-fold (3/3Hbs) or the rather recently discovered 2-on-2 α-helical fold (2/2Hbs) (3). The phylogenetic complexity of Hbs is equaled by the number of reactions catalyzed by the hemoproteins (4). These include O2 transport and storage also in nonanimal organisms (5, 6) as well as sulfide transport and the detoxification of nitric oxide (NO), nitrite, and peroxides (4). As most Hbs are able to catalyze several or all of the possible reactions in vitro, biochemical studies are not sufficient to explain Hb functions in vivo (7). Therefore, the physiological functions of many Hbs remain unknown, and the cellular role of 2/2Hbs has only been analyzed in a few species (8). In this study, we analyzed the function of a 2/2Hb encoding gene in the unicellular green alga Chlamydomonas reinhardtii. Chlamydomonas, although in the plant kingdom, has retained many genes from the common ancestor of both plants and animals, which is why it serves also as a model for ciliary diseases (9, 10). In addition, there are sets of genes related to those in bacteria, especially dealing with anaerobic pathways (11), including hydrogenases of the [FeFe]-type responsible for hydrogen gas production in anaerobic C. reinhardtii cells (12, 13).
RNA-Seq analyses of anaerobic algae revealed a transcript, Cre16.g661200, whose amounts increased more than 1,000-fold from hardly detectable levels in the control (aerated, illuminated) culture. Cre16.g661200 encodes a protein with an N-terminal 2/2Hb domain, which we named truncated hemoglobin 8 (THB8). Here, we show that the THB8 gene is needed for hypoxic growth as well as for proper induction of some anaerobic genes. Posttranscriptional silencing of THB8 results in strains unable to grow hypoxically in the presence of a chemical NO scavenger. As recombinant THB8 protein interacts with NO and this second messenger also influences growth and gene expression in Chlamydomonas wild-type cells, we postulate that THB8 is involved in an NO-dependent signaling cascade essential for anaerobic acclimation of the cells.
Results and Discussion
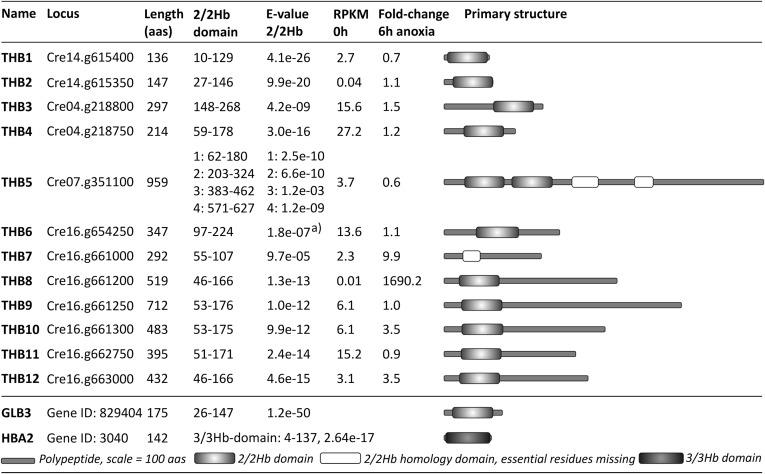
Transcriptome profiles obtained by RNA sequencing revealed two transcripts, encoding proteins belonging to the 2/2Hb family, that accumulated more than fourfold in anaerobic versus aerobic C. reinhardtii cells (Fig. 1), and two that accumulated 3.5-fold. Cre16.g661200, termed THB8 in this study, showed particularly high fold changes. It was hardly detectable in noninduced cells [having an estimated expression of about 0.005 reads per kb of mappable transcript length per million mapped reads (RPKM)], but became the most abundant 2/2Hb encoding transcript in anaerobic cells (69.7 RPKM). Therefore, a role of this 2/2Hb in the anaerobic response seemed likely.
Fig. 1.
Twelve putative 2/2Hb-encoding genes can be found in the C. reinhardtii genome. Basic features of the encoded proteins are summarized. The 2/2Hb domain and E-value 2/2Hb indicate positions and similarity E-values of predicted 2/2Hb domains. “RPKM 0h” represents the estimated expression in units of reads per kb of mappable transcript length per million mapped reads (RPKMs) in light-grown cultures. “Fold-change 6h anoxia” represents the change of transcript abundance relative to the control (0 h) after 6 h of anaerobiosis. Schematic overviews of the THB polypeptide primary sequences are depicted to the right. Arabidopsis GLB3 (hemoglobin 3) and human HBA2 (hemoglobin, alpha 2) are shown at the bottom. THB1-4 were named before (9); THB5-12 were named in this study. Positions of 2/2Hb domains are according to sequence alignments within the CD-database (cd00454) on the National Center for Biotechnology Information (NCBI) database or by the InterProScan pfam-method in case of the incomplete third and fourth domains of THB5 and the single domain of THB7. E-values were inferred by NCBI/CD search, Bacterial-like globin (pfam01152), using only the 2/2Hb domain. The superscript “a” indicates that No pfam-domain was detected. The depicted E-value is indicated in the CD-alignment (cd00454: Trunc_globin domain).
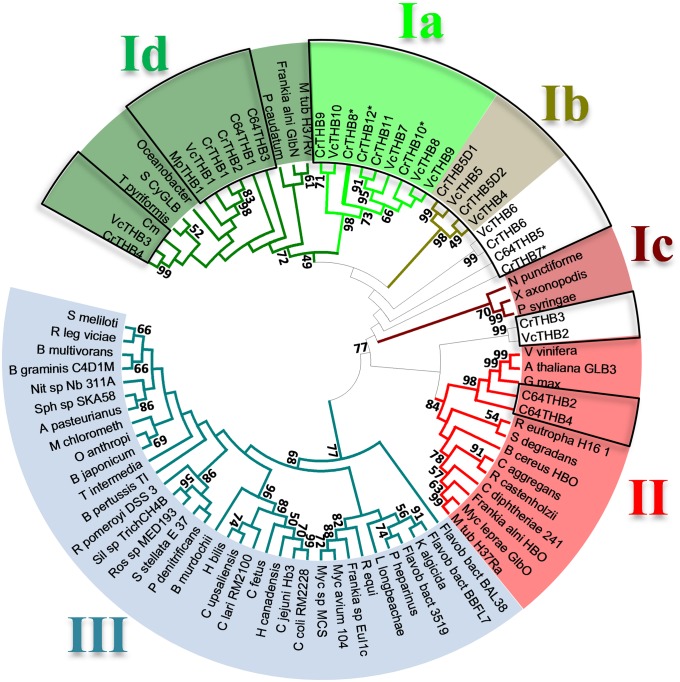
We first used several known 3/3Hb and 2/2Hb sequences (Table S1) as queries in BlastP analyses on Phytozome (C. reinhardtii v4.3 on Phytozome v8.0) to update the previous inventory (14). We identified 12 putative 2/2Hb-encoding genes in Chlamydomonas and none encoding 3/3Hbs (Figs. 1 and 2). Each protein clusters with known eukaryotic and prokaryotic class I 2/2Hb (2/2Hb-I) proteins such as the well-studied globin LI410 from Chlamydomonas moewusii (3). However, five C. reinhardtii 2/2Hbs, as well as 4 out of 10 orthologs detected in the Volvox carteri genome (Tables S1 and S2), constitute a Chlorophycean-specific subclass of 2/2Hb-I proteins, which we termed 2/2Hb-Ia (Fig. 2). Their 2/2Hb domains share identity levels between 54% and 94% and a group-specific sequence motif of 27–28 amino acids downstream of the 2/2Hb domain (positions 155–180 in THB8) with the consensus sequence (K/R)xxP(F/Y)KDAIFTPSAxDAxEEARWAxE (standard and underlined letters: >60% and 100% conserved, respectively; letters in parentheses: two variations; x: more than two variations). A further characteristic of the C. reinhardtii 2/2Hb family is the unprecedented variety of domains and lengths. For example, the C terminus of isoform THB8 comprises two thirds of the whole sequence but lacks any known domains or motifs. Eight out of nine chlorophycean 2/2Hb sequences of subclass Ia feature an elongated and unknown C-terminal part (Fig. 1).
Fig. 2.
C. reinhardtii THB8 belongs to a Chlorophycean subgroup of 2/2Hbs. The phylogram shows the evolutionary relationships of class I, II, and III 2-on-2 hemoglobins and the subgroup I a, which includes THB8. The evolutionary history was inferred using the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method (49). The optimal tree with the sum of branch length = 33.07167863 is shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches for values ≥50. The phylogram is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are given in units of number of amino acid substitutions per site. The 2/2Hb-domain regions of 86 representative amino acid sequences were analyzed (see Table S2). All positions containing gaps and missing data were eliminated. There were 81 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 (50). Colored boxes mark different 2/2Hb classes and subclasses correspondingly labeled with roman numbers I–III. The 2/2Hbs from Chlorophyta are indicated by open boxes. The * indicates C. reinhardtii 2/2Hbs whose transcripts accumulate upon anaerobiosis.
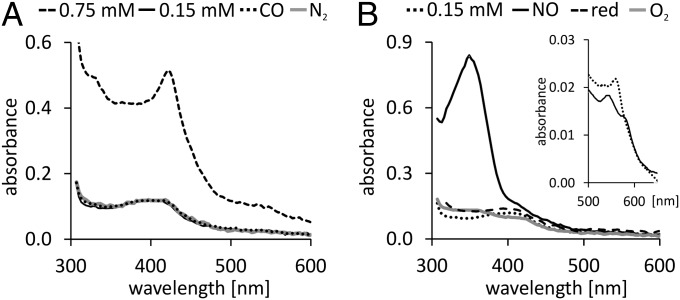
The THB8 protein was produced heterologously to verify that it indeed binds heme-like typical hemoglobin. UV-Vis spectroscopic analyses of recombinant THB8 revealed a Soret-band at λ = 420 nm (Fig. 3A), typical for hemoproteins, as well as a peak at λ = 537 nm and a shoulder at λ = 567 nm reported for the ferric forms of some 2/2Hbs (15–17). The spectra did not change significantly upon treating the aerobically isolated THB8 samples with N2 or CO (Fig. 3A). Most of the Hbs studied to date interact with NO, and NO itself is a second messenger in multiple regulatory networks. In an NO/N2 atmosphere, the degassed ferric form of THB8 (Inset in Fig. 3B) exhibited a distinctive peak at 350 nm (Fig. 3B). Specific absorption maxima of Hbs at 350 nm have only rarely been reported in literature. A peak around 350 nm, however, has been correlated with nitrosylation of proteins, which particularly occurs at exposed thiols (18). It was demonstrated that an absorption maximum of human myoglobin(Mb) at 350 nm was due to S-nitrosylation of a specific Cys residue (19). The THB8 polypeptide contains 10 Cys residues, 2 of which are present in its 2/2Hb domain. The 350-nm signal of NO-treated THB8 was reversible by adding the reductant sodium dithionite (Fig. 3B), also speaking for the signal being due to S-nitrosothiol formation (20). Most heme-catalyzed reactions resulting in S-nitrosothiol formation occur in the presence of oxygen, usually upon reactions of oxy-ferrous Hb or Mb (4, 19, 21). However, the reaction of ferric human Hb with NO and subsequent S-nitrosothiol formation is possible, too (22), and a similar mechanism might be operable in recombinant THB8. Finally, a heme-independent reaction of functional groups of the THB8 apo-protein with NO is a possible scenario, in analogy to S-nitrosothiol formation in human apo-myoglobin (19).
Fig. 3.
THB8 is a hemoprotein that reacts with NO. (A and B) UV-Vis absorption spectra of recombinant THB8 recorded at a path length of 1 mm. (A) Spectra of the aerobically isolated protein (0.75 or 0.15 mM) before and after purging with N2 or CO. Note that the lower concentration was used for all spectra in which the protein solution was treated with a gas or a reductant to make the spectra comparable with the NO-treated sample. (B) Spectra of 0.15 mM degassed THB8 before and after treatment with NO. The resulting absorption maximum at λ = 350 nm was reversed by addition of the reducing agent sodium dithionite (indicated as “red”) or by adding aerated water (indicated as “O2”). The Inset in B shows the alpha bands of the as isolated (ferric) (solid line) and the sodium dithionite reduced (deoxy) (dotted line) THB8 protein.
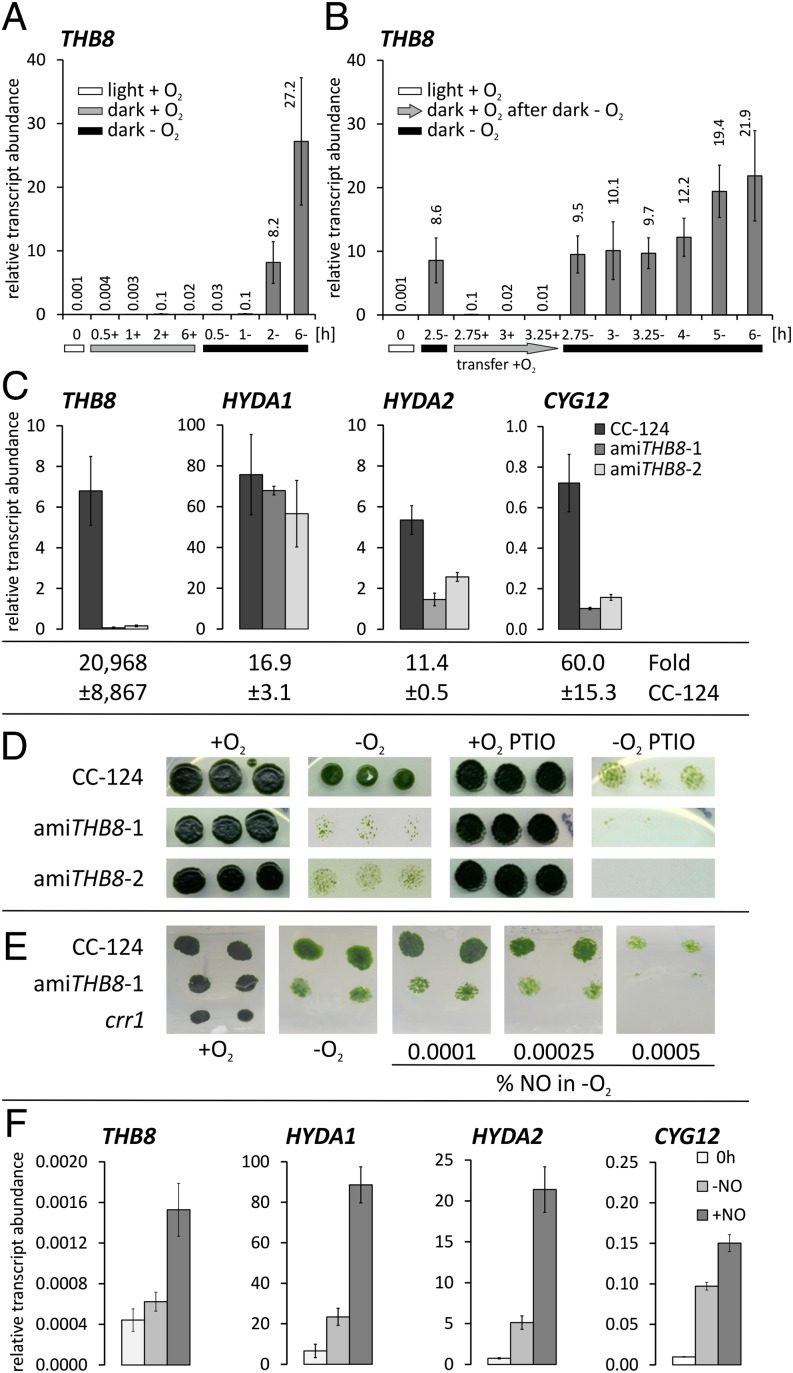
The stringent regulation of THB8 transcript abundance in anaerobic versus aerated C. reinhardtii cells suggested a prominent function of this 2/2Hb isoform in the anoxic response of the alga. However, the experimental set-up applied in the RNA-Seq project also involved a transition from light to dark conditions, as anaerobiosis was achieved by transferring light-grown precultures to sealed flasks in the dark (see details in Materials and Methods). Therefore, we carried out two types of control experiments to assess the influence of darkness alone and the accumulation of the THB8 transcript as a specific response to the absence of O2, respectively. During the first type of control experiments, illuminated aerobic algal cultures were transferred to open beakers in the dark and stirred well to ensure continued air-saturated conditions. Culture aliquots incubated in sealed flasks served as anaerobic controls (Fig. 4A). Results obtained by quantitative real-time PCR (qPCR), using RNA isolated at various time points of dark-oxic and dark-anoxic cells, showed that THB8 transcript abundances increased significantly only in anaerobic C. reinhardtii cultures (Fig. 4A). In the second type of control analyses, light-grown C. reinhardtii cultures were first transferred to sealed flasks in the dark to create anaerobic conditions and to obtain enhanced THB8 transcript amounts (Fig. 4B). After 2.5 h, three of the sealed flasks were opened, and the cells were poured into open beakers and incubated upon stirring in the dark for 15, 30, and 45 min before isolating RNA (Fig. 4B). THB8 transcript amounts in these reaerated cultures were compared with those determined in cultures kept in sealed flasks and maintained in anaerobiosis, respectively, in the dark (Fig. 4B). This set-up revealed that the amount of THB8 transcripts detectable after 2.5 h of anaerobiosis decreased almost to the noninduced level within 15 min of reaeration in the dark whereas the enhanced level was maintained in cells kept in anaerobiosis (Fig. 4B). The results of both experiments showed that accumulation of THB8 transcript was specific to the absence of O2, and that anoxia was required to maintain high THB8 transcript amounts (Fig. 4 A and B). However, although the general pattern we observed was a gradual increase of THB8 transcript abundance during prolonged incubation under dark-anoxic conditions, we noticed a marked variability of THB8 transcript abundances during these analyses and further experiments (see below). This variability indicates that the regulation of THB8 gene expression is sensitive to even moderate variations of the cell culture status.
Fig. 4.
THB8 is required for NO-dependent hypoxic growth and induction of anaerobic genes. (A) Aerobically and illuminated grown cells (0 h) were incubated for the indicated time points in open (+) or sealed (−) flasks in the dark. The bars at the bottom indicate the following: white, light/aerobiosis (+O2); gray, dark/aerobiosis (+O2); black, dark/anaerobiosis (−O2). (B) Cultures were incubated for 2.5 h anaerobically in the dark (2.5−) and then transferred to open beakers in the dark (+, indicated by a gray arrow) or kept in anaerobiosis (−, indicated by a black bar) in the dark for the indicated time points in hours. (C) Wild-type CC-124 and transformants amiTHB8-1 and -2 were grown aerated in the light and then incubated anaerobically in the dark for 7 h. RNA was extracted from the precultures and after dark–anoxic incubation. Below the individual graphs, fold-changes of the individual transcripts in the wild type, calculated using the ΔΔC(t) method related to values obtained from preculture RNA, are depicted. (D) Strain CC-124 and amiTHB8-1 and -2 grown without or with 100 µM PTIO. Each plate with or without PTIO was incubated under aerobic (+O2) or hypoxic (−O2) conditions in the light. (E) Cells were grown on TAP agar in flasks. Anaerobic conditions were achieved by N2 purging of gas tightly sealed vessels. NO was added as NO/N2 mixture. The crr1 mutant, which does not grow under hypoxic conditions (41), was used as control for maintained anaerobiosis. (D and E) The result of one experiment is depicted. The same results were obtained by two repetitions. (F) Light-grown C. reinhardtii cultures (0 h) were transferred to two open beakers in the dark, and DEA-NONOate was added to one culture to a final concentration of 10 µM (+NO). RNA was isolated after 45 min. (A, B, C, and F) Transcript abundances relative to RCK1, whose amounts stay constant during anaerobiosis, were determined by qPCR and multiplied by 1,000. (A and B) Averages ± SD of three independent experiments are shown. (C and E) The average of two independent analyses is depicted; error bars indicate the SD.
For the analysis of the function of the THB8 gene, knock-down strains exhibiting reduced THB8 transcript amounts were generated applying the artificial microRNA (amiRNA) technology established by Molnár et al. (23). Two C. reinhardtii strains, amiTHB8-1 and amiTHB8-2, were isolated. After 7 h of anaerobic incubation in the dark, the corresponding strains had only 0.9% and 2.3%, respectively, of THB8 transcript abundance determined in the parental strain CC-124 (Fig. 4C). Fold-changes compared with aerated light-grown controls were 0.8% and 3.4% of the fold-changes (20,968-fold ± 8867) detected in the wild type (Fig. S1A). Both transformants exhibited anaerobically induced hydrogenase activity (Fig. S1B) as well as O2-exchange rates (Fig. S1C) similar to the parent. We concluded that neither a disturbance of the major energy-generating pathways nor a general unresponsiveness to anoxia resulted from THB8 silencing and that data obtained examining the transformants would be characteristic for the mis-regulation of the THB8 gene.
We used qPCR to monitor the abundances of 22 anaerobically accumulating transcripts and noted only moderate differences in the expression patterns of most of these genes (Table S3). Transcription of the HYDA1 gene, encoding the major [FeFe]-hydrogenase isoform of C. reinhardtii (24, 25) and inducible by anaerobiosis (26), was similar in strains amiTHB8-1 and -2 vs. strain CC-124 (Fig. 4C). In contrast, the abundances of HYDA2 transcripts were only about 30% (amiTHB8-1) and 50% (amiTHB8-2) (Fig. 4C) compared with wild-type amounts. HYDA2 encodes a second [FeFe]-hydrogenase HYDA2 of C. reinhardtii (25), which contributes to about 25% of maximal hydrogenase enzyme activity in anaerobic algal cells (24). The physiological role of HYDA2 is unclear. A mutant deficient for HYDA1 has much lower H2-production rates in the light whereas dark H2 production is not impaired in this strain (24). It might be speculated that HYDA2 becomes more important during fermentative H2 generation in the dark. More pronounced differences of transcript fold-changes in amiTHB8-strains versus wild type were observed for CYG12. The CYG12 gene encodes a soluble guanylyl cyclase (sGC) (27, 28). sGCs of mammals are heme-containing heterodimeric NO sensors that are activated upon binding of NO to the heme group (29). In the activated form, sGCs convert GTP to cGMP, a second messenger taking part in the regulation of a variety of cell functions. CYG12 transcripts accumulated up to 60-fold upon anaerobiosis in the C. reinhardtii wild type. AmiTHB8 transformants 1 and 2 had 14% and 22% of CYG12 relative transcript abundance after 7 h of anaerobiosis (Fig. 4C). In addition to the reactivity of recombinant THB8 toward NO, the aberrant expression of a gene related to NO signaling in THB8 knockdown strains was a further indicator that the THB8 protein might be involved in an NO-dependent pathway active in anaerobic C. reinhardtii cells.
Posttranscriptional silencing of THB8 had a severe effect on cell growth on Tris Acetate Phosphate (TAP) agar plates under hypoxic conditions in the light [at 50 µmol of photons⋅m−2⋅s−1 photon flux density (PFD)] (Fig. 4D). Liquid cultures of strains amiTHB8-1 and -2 incubated in dim light (15 µmol of photons⋅m−2⋅s−1 PFD) and purged constantly with 0.1% CO2 in N2 did also grow slower than the wild type (Fig. S2A). Under the same conditions, but purged with 0.1% CO2 in air, both amiTHB8 transformants showed a similar growth as the parental strain CC-124 (Fig. S2B). Both 3/3Hbs and 2/2Hbs have been reported to act as NO dioxygenases (7, 8, 30). To investigate whether C. reinhardtii THB8 might act as an NO scavenger and whether the hypoxic growth defect of the amiTHB8 strains resulted from NO overaccumulation, respectively, the cells were grown on agar plates in the presence of the specific NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO) (31). However, hypoxic growth of amiTHB8-1 and -2 was then completely abolished (Fig. 4D). This compound also blocked growth in hypoxic wild-type cells (Fig. 4D). As neither strain was affected by PTIO under aerobic conditions, the observed growth defects were probably specifically due to the diminution of NO levels within hypoxic cells. Three conclusions can be drawn from these observations. First, the physiological function of THB8 is not to protect C. reinhardtii from NO overaccumulation. Second, NO is important for hypoxic growth of C. reinhardtii. Third, proper expression of the THB8 gene is needed for this NO-dependent hypoxic growth. In view of the above described results, the latter suggests that THB8 might react with the NO molecule and deliver it to required signal transduction pathways. The poor, but significant, growth of the C. reinhardtii wild type would result from the presence of sufficient amounts of THB8 proteins competing with the chemical NO scavenger and providing a limited amount of NO to NO-dependent pathways. In a second hypothesis, THB8 itself might be a component of a signaling pathway and be activated by NO. This hypothesis was supported by experiments in which the strains were grown in an anaerobic atmosphere containing low concentrations of NO (Fig. 4E). Strain amiTHB8-1 was not rescued by the presence of external NO, indicating that the phenotype cannot be bypassed simply by enhancing the concentration of the second messenger. Notably, higher concentrations of NO (0.0005 vol-%) significantly impaired growth of the strains (Fig. 4E), indicating the need for a tight control of intracellular levels of the potentially cytotoxic radical NO (32, 33).
To gain further insights into the NO effect on the hypoxic response of C. reinhardtii, the influence of the chemical NO donor DEA-NONOate [2-(N,N-diethylamino)-diazenolate 2-oxide] on transcript abundances of THB8, HYDA1, HYDA2, and CYG12 in aerobic algal cultures was determined. Externally added NO induced the transcription of these genes despite the presence of O2 to different extents. The transcript abundances of the HYDA1 and HYDA2 genes were the most affected and showed similar amounts as determined in anaerobic cells (Fig. 4F). THB8 and CYG12 transcript abundances were 2.5-fold and 1.5-fold enhanced in NO-treated vs. untreated cells although their amounts stayed significantly below those reached under anaerobiosis (compare Fig. 4 F and C). The results show that the expression of “anaerobic genes” in C. reinhardtii can be triggered by NO in the absence of the natural stimulus anaerobiosis, suggesting that the cellular signal transduction cascade(s) involve(s) the NO molecule.
The results of this study report that NO plays a role in the hypoxic response of the green alga C. reinhardtii, which is important for the cells to adapt to and grow under conditions of O2 limitation. The mechanisms of NO generation in the microalga are yet under research, but two possibilities have been proposed. Nitrite-dependent NO production, absent in a nitrate reductase mutant (34), suggests a nitrate reductase-dependent process like that in higher plants (32, 35). NO synthesis in C. reinhardtii supplied with ammonium versus nitrate is sensitive to the Arg analog Nω-Nitro-l-arginine methyl ester (l-NAME) (28), an inhibitor of mammalian NO synthases. Because the C. reinhardtii wild type used in this study is a natural nitrate reductase mutant, the former reaction cannot be responsible for hypoxic NO generation in this strain. In plants, a variety of NO-producing reactions, including the respiratory chain, have been described and probably differ depending on the organ and the environmental stimulus (35). In Chlamydomonas, NO has been shown to be also involved in the acclimation of the alga to growth on ammonium versus nitrate (28) or to copper stress (36), indicating that NO-based signaling pathways in Chlamydomonas are as versatile as in higher plants (35) or mammals (37).
The C. reinhardtii 2/2Hb THB8 is required for proper hypoxic growth and is involved in the activation of genes in response to anaerobiosis. As THB8 interacts with NO, THB8-knockdown strains cannot grow hypoxically when NO is scavenged by PTIO, and NO itself can trigger transcript accumulation of otherwise anaerobically induced genes, we suggest that THB8 participates in an NO-dependent signaling pathway operative upon oxygen limitation in C. reinhardtii. Reactions of Hbs with the NO molecule are manifold and have roles in NO scavenging, fermentation, and signaling (4, 21, 30, 38). The THB8 protein might trigger responses upon its reaction with NO and could be a yet unclassified mediator between the NO signal and the cellular responses reported here. The long C terminus of THB8 suggests the presence of a function of the protein in addition to ligand binding and/or conversion. The findings presented in this work therefore contribute to the understanding of the largely unexplored complexity of Hb functions and open up new directions of Hb and NO signaling research.
Materials and Methods
Organisms and Growth Conditions.
C. reinhardtii strain CC-124 wild-type mt− [137c] [nit1 (nitrate reductase) nit2 (encoding a transcription factor regulating nitrogen metabolism) agg1 (allele for phototactic aggregation)] was obtained from the Chlamydomonas Resource Center (Department of Plant Biology, University of Minnesota) and grown photoheterotrophically in Tris Acetate Phosphate (TAP) medium (39) at a light intensity of 100 µmol of photons·m−2·s−1 at 20 °C. Cell numbers were determined using a hemocytometer. Chlorophyll concentrations were analyzed by acetone extraction as described before (40).
Analyses of the responses of the cells to O2 limitation were done in different set-ups. To obtain truly anaerobic conditions without a possible interference of O2 produced by photosynthetic activity, cell suspensions grown aerated in the light (referred to as “0 h” throughout the text) were transferred to sealed flasks in the dark. Cells were first grown to early-logarithmic growth phase (ca. 3 × 106 cells·mL−1). One hundred milliliters of culture aliquots were then transferred to squared glass bottles sealed with Red Rubber Suba Seals 37 (Sigma-Aldrich), which had a total volume of 118 mL after insertion of the Suba Seals. The flasks were subsequently incubated in a dark room on magnetic stirrers at 20 °C. After the time points indicated in the text, the cell suspensions of individual flasks were collected for RNA extraction. Reference RNA was isolated from 100-mL culture aliquots removed from the illuminated and aerated precultures. As darkness alone can influence gene expression of a photosynthetic organism, control experiments were conducted in which 100-mL aliquots of the aerated and light-grown cells were transferred to darkness and incubated in 500-mL beakers loosely covered with aluminum foil. RNA was isolated from these cells, which were well-aerated by stirring, at the time points indicated in the text. The impact of reaerating anaerobic cells was also analyzed. For these experiments, 100-mL aliquots of the algal suspension were incubated for 2.5 h in sealed flasks in the dark as described above. Then, three flasks were opened, and the cells were transferred to open 500-mL beakers. RNA was isolated from these cultures after 15, 30 and 45 min, as well as from cells that were maintained in sealed flasks, and THB8 mRNA abundance was measured by qPCR.
For analyzing growth of the C. reinhardtii wild type and transformants amiTHB8-1 and -2, two types of experiments were conducted. In the first set-up, growth on TAP agar at a light intensity of 50 µmol of photons·m−2·s−1 at 20 °C was analyzed. All strains were grown in liquid TAP medium as described above until the early-exponential growth phase (ca. 6 µg of chlorophyll·mL−1). Then the cell suspensions were diluted to 1 µg of chlorophyll·mL−1 with fresh TAP medium in sterile 1.5-mL reaction tubes. Ten microliters of these dilutions were spotted in triplicates on two TAP agar plates. One of the plates was placed in a gas-tight bag with the anaerobic generator from the “GENbag anaer” system from Biomérieux whereas the other plate was incubated under aerobic conditions as control. When indicated, the selective NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO, Sigma Aldrich) dissolved in ethanol was added to the agar to a final concentration of 100 µM. To test the effect of externally added NO on hypoxic growth of C. reinhardtii strains, cell suspensions were dropped on TAP agar in flasks. Anaerobic conditions were established by N2 purging. When indicated, the respective concentration of NO gas was achieved by injecting a mixture of NO in N2 instead of applying a chemical NO donor. This proceeding was chosen because most chemical NO donors have only short half-lives. As the setup did not include the O2-removing generator of the GENbag anaer system (Biomerieux), a crr1 (COPPER RESPONSE REGULATOR 1) mutant was used as a control for hypoxia. The crr1 mutant is unable to grow under hypoxic conditions (41). The impaired growth of amiTHB8-transformants was further analyzed in liquid cultures in a set-up similar to one described earlier (41), but modified as follows: Chlamydomonas cultures were inoculated at a chlorophyll concentration of 0.1 µg of Chl⋅mL−1 in 100 mL of TAP medium in squared glass bottles. These were sealed with Suba Seals and incubated on a magnetic stirrer in dim light (15 µmol of photons⋅s−1⋅m−2). The cell suspension was continuously purged with 0.1% CO2 in N2. After the indicated time points, samples were withdrawn for determining the chlorophyll content.
RNA Isolation and qPCR.
Isolation of total RNA, cDNA synthesis, and qPCR analyses were done as described previously in detail (42). Briefly, total RNA was first treated with DNase (TURBO DNase from Ambion/Applied Biosystems) according to the manual. Afterward, cDNA was synthesized from DNase-treated RNA using oligo(dT18) oligonucleotides and M-MLV (Moloney murine leukemia virus) Reverse Transcriptase (Invitrogen) as recommended by the manufacturer. qPCR reactions were performed with the peqGOLD Taq DNA Polymerase Kit (PeqLab) using Buffer S and 5× Enhancer solution included in the kit. Each sample was analyzed in technical triplicate using DNA Engine Opticon 2 from MJ Research. Transcript levels of CBLP (Chlamydomonas beta subunit-like polypeptide)/RCK1 (receptor of activated protein kinase C) (Cre13.g599400), which were constant in all samples, were used as a reference to calculate relative transcript abundances (43). qPCR oligonucleotides are listed in Table S4.
RNA Sequencing and Bioinformatics.
RNA for RNA-Seq analyses was obtained from cells grown under aerobic conditions in the light until a density of 2.2 × 106 cells⋅mL−1 (0 h), which were then transferred to sealed flasks in the dark to establish anaerobic conditions due to the respiratory activity of the cells. RNA was isolated from the reference culture (0 h) and from cells incubated for 6 h in sealed flasks. Total RNA was treated with DNase as described above and was sequenced at the Joint Genome Institute (JGI) using the Illumina platform. About 20 million 76-bp reads were obtained per sample. Sequence files are publicly available at NCBI’s Gene Expression Omnibus (accession no. GSE42035). Reads were aligned to the Au10.2 transcripts sequences (v4.3 assembly of the Chlamydomonas genome) using Bowtie (44) in single-end mode and with a maximum tolerance of three mismatches. Expression estimates were obtained for each sample in units of reads per kb of mappable transcript length per million mapped reads (RPKMs) (45) after normalization by the number of aligned reads and mappable transcript length (computed for 76-mers) as described earlier (46). Fold-changes were calculated from the average between replicates. Differential expression analysis was performed using the DESeq package (47), and false discovery rates (FDRs) were estimated in R with the Benjamini–Hochberg correction (48). Genes were selected that showed a fold regulation greater than 4 (FDR below 5%).
THB8 Gene Silencing.
For generation of amiTHB8-transformants, we used vector pChlamiRNA3int (23) and the specific amiRNA TAAATCTTCACTAATTCACTG. The construct was generated according to the instructions given in Molnár et al. (23). Transformation of C. reinhardtii strain CC-124 was conducted according to the glass-bead method after removal of the cell wall by autolysin.
Heterologous Expression.
The C. reinhardtii THB8 (Cre16.g661200) coding sequence was amplified using oligonucleotides designed by Primer D'Signer 1.1 (IBA) (5′-ATGGTAGGTCTCAAATGGGAAACAGCTGCACCACCTTTG-3′, 5′-ATGGTAGGTCTCAGCGCTGGTCATGCGCAGGCGTGACG-3′) and ligated via BsaI with pASK-IBA3 plus (IBA). Transformation of Escherichia coli BL21(DE3)pLysS (Novagen/Merck) and all further steps were done according to the manual of IBA. UV-Vis absorption spectra were recorded using a NanoDrop 1000 (PeqLab) spectrophotometer. The effect of gases was examined by purging the as isolated (ferric) protein solution for 10 min with N2, carbon monoxide (CO) (99,997%), or air. NO was added as a 10% NO in N2 mixture to protein solutions degassed with N2. Reduction of the THB8 protein was achieved by adding 10 µM sodium dithionite under anaerobic conditions.
Generation of the Phylogenetic Tree Shown in Fig. 2.
The phylogenetic tree was created following the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) method (49) using MEGA5 (50). The tree is drawn to scale, with branch lengths corresponding to calculated evolutionary distances between 86 proteins. Bootstrap support values are based on 1,000 bootstrap replicates and are presented for values ≥50.
Supplementary Material
Acknowledgments
We thank Astrid Weber and Dennis Huwald for technical support. RNA-samples were sequenced at the United States Department of Energy Joint Genome Institute, which is supported by the Office of Science of the US Department of Energy under Contract DE-AC02-05CH11231. A.H. was funded by Deutsche Forschungsgemeinschaft Grant He 5790/1-1. The work was further supported by the Deutsches Zentrum für Luft- und Raumfahrt (Modular Life Support and Energy System) (T.H.) and National Institutes of Health Grant GM42143 (to S.S.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE42035).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302592110/-/DCSupplemental.
References
- 1.Vázquez-Limón C, Hoogewijs D, Vinogradov SN, Arredondo-Peter R. The evolution of land plant hemoglobins. Plant Sci. 2012;191-192:71–81. doi: 10.1016/j.plantsci.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Vinogradov SN, et al. A phylogenomic profile of globins. BMC Evol Biol. 2006;6:31. doi: 10.1186/1471-2148-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesce A, et al. A novel two-over-two alpha-helical sandwich fold is characteristic of the truncated hemoglobin family. EMBO J. 2000;19(11):2424–2434. doi: 10.1093/emboj/19.11.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinogradov SN, Moens L. Diversity of globin function: Enzymatic, transport, storage, and sensing. J Biol Chem. 2008;283(14):8773–8777. doi: 10.1074/jbc.R700029200. [DOI] [PubMed] [Google Scholar]
- 5.Vigeolas H, Hühn D, Geigenberger P. Nonsymbiotic hemoglobin-2 leads to an elevated energy state and to a combined increase in polyunsaturated fatty acids and total oil content when overexpressed in developing seeds of transgenic Arabidopsis plants. Plant Physiol. 2011;155(3):1435–1444. doi: 10.1104/pp.110.166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakabayashi S, Matsubara H, Webster DA. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature. 1986;322(6078):481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- 7.Smagghe BJ, Trent JT, 3rd, Hargrove MS. NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo. PLoS ONE. 2008;3(4):e2039. doi: 10.1371/journal.pone.0002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouellet H, et al. Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc Natl Acad Sci USA. 2002;99(9):5902–5907. doi: 10.1073/pnas.092017799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merchant SS, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318(5848):245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall WF. Basal bodies platforms for building cilia. Curr Top Dev Biol. 2008;85:1–22. doi: 10.1016/S0070-2153(08)00801-6. [DOI] [PubMed] [Google Scholar]
- 11.Hemschemeier A, Jacobs J, Happe T. Biochemical and physiological characterization of the pyruvate formate-lyase Pfl1 of Chlamydomonas reinhardtii, a typically bacterial enzyme in a eukaryotic alga. Eukaryot Cell. 2008;7(3):518–526. doi: 10.1128/EC.00368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemschemeier A, Happe T. Alternative photosynthetic electron transport pathways during anaerobiosis in the green alga Chlamydomonas reinhardtii. Biochim Biophys Acta. 2011;1807(8):919–926. doi: 10.1016/j.bbabio.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Stripp ST, et al. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc Natl Acad Sci USA. 2009;106(41):17331–17336. doi: 10.1073/pnas.0905343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinogradov SN, Fernández I, Hoogewijs D, Arredondo-Peter R. Phylogenetic relationships of 3/3 and 2/2 hemoglobins in Archaeplastida genomes to bacterial and other eukaryote hemoglobins. Mol Plant. 2011;4(1):42–58. doi: 10.1093/mp/ssq040. [DOI] [PubMed] [Google Scholar]
- 15.Thorsteinsson MV, et al. A cyanobacterial hemoglobin with unusual ligand binding kinetics and stability properties. Biochemistry. 1999;38(7):2117–2126. doi: 10.1021/bi9819172. [DOI] [PubMed] [Google Scholar]
- 16.Couture M, Guertin M. Purification and spectroscopic characterization of a recombinant chloroplastic hemoglobin from the green unicellular alga Chlamydomonas eugametos. Eur J Biochem. 1996;242(3):779–787. doi: 10.1111/j.1432-1033.1996.0779r.x. [DOI] [PubMed] [Google Scholar]
- 17.Watts RA, et al. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc Natl Acad Sci USA. 2001;98(18):10119–10124. doi: 10.1073/pnas.191349198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon DI, et al. Polynitrosylated proteins: Characterization, bioactivity, and functional consequences. Proc Natl Acad Sci USA. 1996;93(10):4736–4741. doi: 10.1073/pnas.93.10.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witting PK, Douglas DJ, Mauk AG. Reaction of human myoglobin and nitric oxide: Heme iron or protein sulfhydryl (s) nitrosation dependence on the absence or presence of oxygen. J Biol Chem. 2001;276(6):3991–3998. doi: 10.1074/jbc.M005758200. [DOI] [PubMed] [Google Scholar]
- 20.Schreiter ER, Rodríguez MM, Weichsel A, Montfort WR, Bonaventura J. S-nitrosylation-induced conformational change in blackfin tuna myoglobin. J Biol Chem. 2007;282(27):19773–19780. doi: 10.1074/jbc.M701363200. [DOI] [PubMed] [Google Scholar]
- 21.Reeder BJ. The redox activity of hemoglobins: From physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13(7):1087–1123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger BP, et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci USA. 2003;100(2):461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molnár A, et al. Highly specific gene silencing by artificial microRNAs in the unicellular alga Chlamydomonas reinhardtii. Plant J. 2009;58(1):165–174. doi: 10.1111/j.1365-313X.2008.03767.x. [DOI] [PubMed] [Google Scholar]
- 24.Meuser JE, et al. Genetic disruption of both Chlamydomonas reinhardtii [FeFe]-hydrogenases: Insight into the role of HYDA2 in H₂ production. Biochem Biophys Res Commun. 2012;417(2):704–709. doi: 10.1016/j.bbrc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Forestier M, et al. Expression of two [Fe]-hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. Eur J Biochem. 2003;270(13):2750–2758. doi: 10.1046/j.1432-1033.2003.03656. [DOI] [PubMed] [Google Scholar]
- 26.Happe T, Kaminski A. Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur J Biochem. 2002;269(3):1022–1032. doi: 10.1046/j.0014-2956.2001.02743.x. [DOI] [PubMed] [Google Scholar]
- 27.Nioche P, et al. Femtomolar sensitivity of a NO sensor from Clostridium botulinum. Science. 2004;306(5701):1550–1553. doi: 10.1126/science.1103596. [DOI] [PubMed] [Google Scholar]
- 28.de Montaigu A, Sanz-Luque E, Galván A, Fernández E. A soluble guanylate cyclase mediates negative signaling by ammonium on expression of nitrate reductase in Chlamydomonas. Plant Cell. 2010;22(5):1532–1548. doi: 10.1105/tpc.108.062380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koesling D, Russwurm M, Mergia E, Mullershausen F, Friebe A. Nitric oxide-sensitive guanylyl cyclase: Structure and regulation. Neurochem Int. 2004;45(6):813–819. doi: 10.1016/j.neuint.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Perazzolli M, Romero-Puertas MC, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. J Exp Bot. 2006;57(3):479–488. doi: 10.1093/jxb/erj051. [DOI] [PubMed] [Google Scholar]
- 31.Akaike T, et al. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 32.Blokhina O, Fagerstedt KV. Oxidative metabolism, ROS and NO under oxygen deprivation. Plant Physiol Biochem. 2010;48(5):359–373. doi: 10.1016/j.plaphy.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Corpas FJ, et al. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011;181(5):604–611. doi: 10.1016/j.plantsci.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Sakihama Y, Nakamura S, Yamasaki H. Nitric oxide production mediated by nitrate reductase in the green alga Chlamydomonas reinhardtii: An alternative NO production pathway in photosynthetic organisms. Plant Cell Physiol. 2002;43(3):290–297. doi: 10.1093/pcp/pcf034. [DOI] [PubMed] [Google Scholar]
- 35.Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- 36.Zhang LP, Mehta SK, Liu ZP, Yang ZM. Copper-induced proline synthesis is associated with nitric oxide generation in Chlamydomonas reinhardtii. Plant Cell Physiol. 2008;49(3):411–419. doi: 10.1093/pcp/pcn017. [DOI] [PubMed] [Google Scholar]
- 37.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci. 2009;14:1–18. doi: 10.2741/3228. [DOI] [PubMed] [Google Scholar]
- 38.Igamberdiev AU, Hill RD. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J Exp Bot. 2004;55(408):2473–2482. doi: 10.1093/jxb/erh272. [DOI] [PubMed] [Google Scholar]
- 39.Harris EH. The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. San Diego: Academic; 1989. [DOI] [PubMed] [Google Scholar]
- 40.Wellburn A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spetrophotometers of different resolution. J Plant Physiol. 1994;144:307–313. [Google Scholar]
- 41.Eriksson M, et al. Genetic dissection of nutritional copper signaling in chlamydomonas distinguishes regulatory and target genes. Genetics. 2004;168(2):795–807. doi: 10.1534/genetics.104.030460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pape M, Lambertz C, Happe T, Hemschemeier A. Differential expression of the Chlamydomonas [FeFe]-hydrogenase-encoding HYDA1 gene is regulated by the copper response regulator1. Plant Physiol. 2012;159(4):1700–1712. doi: 10.1104/pp.112.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 46.Castruita M, et al. Systems biology approach in Chlamydomonas reveals connections between copper nutrition and multiple metabolic steps. Plant Cell. 2011;23(4):1273–1292. doi: 10.1105/tpc.111.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc, B. 1995;57(1):289–300. [Google Scholar]
- 49.Kalinowski ST. How well do evolutionary trees describe genetic relationships among populations? Heredity (Edinb) 2009;102(5):506–513. doi: 10.1038/hdy.2008.136. [DOI] [PubMed] [Google Scholar]
- 50.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.