Figure 3.
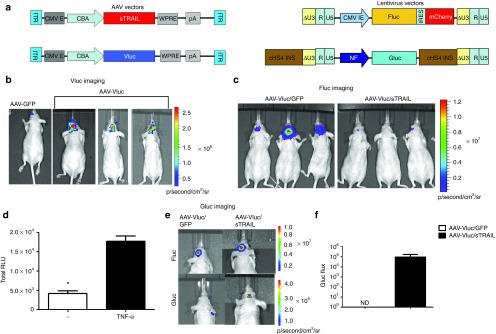
Monitoring of sTRAIL-mediated gene therapy with triple bioluminescence imaging. (a) Schematic diagram for the different AAV (left) and lentivirus (right) vector constructs used in this study. AAV vectors: CMV E, cytomegalovirus enhancer; CBA, chicken β-actin promoter; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; pA, bGH poly A signal. Lentivirus vectors: Diagrams shown represent integrated provirus. CMV IE, cytomegalovirus immediate early promoter; cHS4 INS, chicken β globin hypersensitive site 4 insulator sequence; NF, nuclear factor-κB inducible promoter. (b–f) 105 U87-Fluc/NF-Gluc glioma cells were implanted intracranially in nude mice. Nineteen days later, the mice brains were infused at the same tumor injection site with 1010 gc of either AAV-Vluc/GFP, AAV-Vluc/sTRAIL, or AAV-GFP alone. (b) Vluc bioluminescence imaging performed 10 days after vector injection confirming AAV-mediated gene delivery. (c) Two weeks post-vector injection, tumor volume was imaged by Fluc bioluminescence imaging. (d) Confirmation of NF-Gluc construct. U87-Fluc/NF-Gluc cells in culture were treated with or without tumor necrosis factor-α (TNF-α) (10 ng/ml). Forty-eight hours later, conditioned medium were collected and assayed for Gluc activity to detect levels of NF-κB induction (*P = 0.0001). (e,f) NF-Gluc imaging, a marker for TRAIL binding on glioma cells performed after iv injection of coelenterazine. Calculation of NF-κB activation in glioma-bearing mice treated with AAV-Vluc/sTRAIL or AAV-Vluc/GFP shown in f. Note that no Gluc signal was detected in the AAV-Vluc/GFP mice at any time point. Representative mice from each group are showing in (b,c, and e; n = 5/group). ITR, inverted terminal repeat; nd, not detected.
