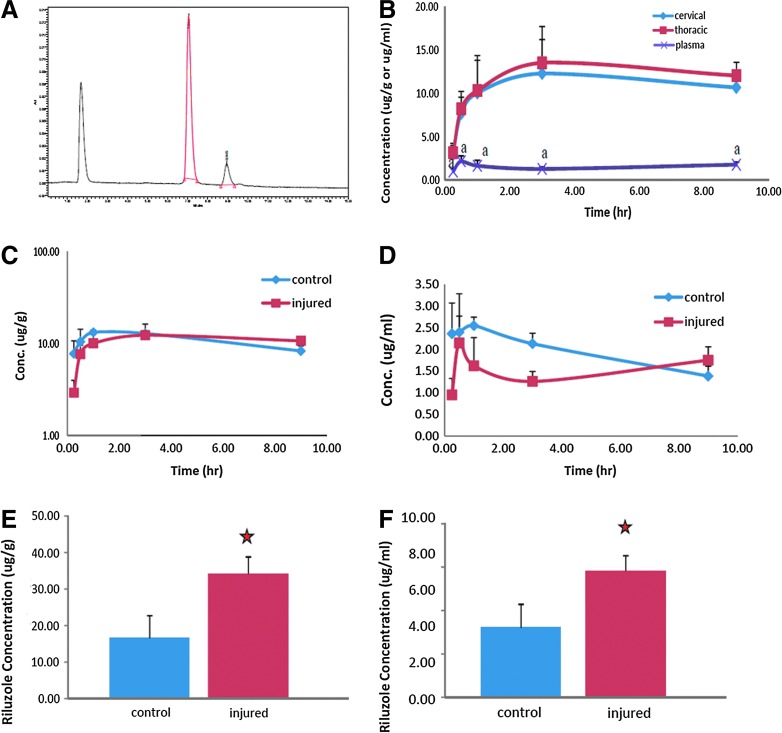
FIG. 8.
Pharmacokinetic profile of riluzole. Representative chromatogram of riluzole (A). Pharmacokinetic profile of riluzole in the plasma and in the cervical and thoracic spinal cord segments after SCI (B). Riluzole in spinal cords reached substantially higher levels than that in plasma with a concentration ratio of 6:1. Riluzole penetrated the spinal cord tissue rapidly and reached concentrations of 8.31±0.96 μg/g at 30 min after injection. The pharmacokinetic characteristics of riluzole were altered by the SCI status (C and D). Estimated half-life increased from 9.2–10.3 h in uninjured controls to 25.1–31.6 hours in SCI rats. The impact of SCI was further confirmed with the spinal cord and plasma samples from the multiple-dosing regimen (E and F). Concentration of riluzole in the spinal cord tissue of control and injured rats after a 3 day treatment protocol (E). Plasma concentration of riluzole was also significantly higher in the injured rats compared to uninjured controls (F).