Table 1.
Conditions for Reaction Kinetics Optimizationa
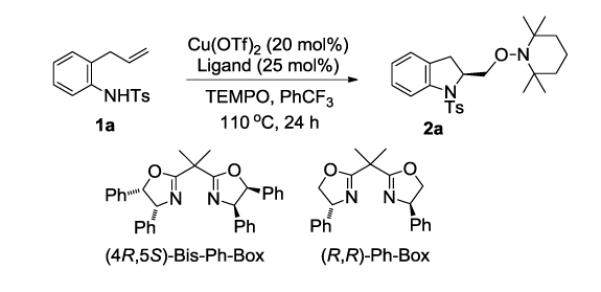
| Entry | Ligand | Base | Yield (%)b | ee (%)c |
|---|---|---|---|---|
| 1 | (4R,5S)-Bis-Ph-Box | K2CO3 | 97 | 90 |
| 2 | (R,R)-Ph-Box | K2CO3 | 90 | 88 |
| 3 | (R,R)-Ph-Box | NBu4OAc | 98 | <5 |
| 4 | (R,R)-Ph-Box | 2,6-di-t-butyl-4-methyl- pyridine |
75 | 87 |
| 5 | (R,R)-Ph-Box | - | 87 | 88 |
| 6d | (R,R)-Ph-Box | - | 88 | 87 |
Cu(OTf)2 (20 mol%), (R,R)-Ph-Box (25 mol%) and PhCF3 (0.07 M w/r to 1a) were combined i a pressure tube and heated to 60 °C for 2 h. Substrate 1a (1 equiv, 0.139 mmol), TEMPO (3 equiv), K2CO3 (1 equiv) were added. The reaction mixture was heated to 110 °C for 24 h.
Yield (%) refers to amount of isolated 2a after purification by flash chromatography on SiO2.
Enantiomeric excesses were determined by chiral HPLC analysis.
The reaction was run using 20 mol% of (R,R)-Ph-Box. OTf = trifluoromethanesulfonyl.
