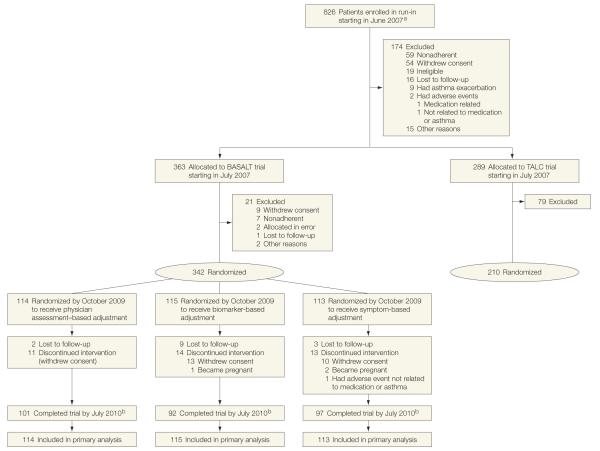
Figure 1.
Participant Allocation in BASALT and TALC Trials
Patients were allocated to the Best Adjustment Strategy for Asthma in the Long Term (BASALT) trial based on achievement of forced expiratory volume in the first second of expiration of greater than 70% during the run-in period and concomitant control of symptoms (score of 0 or 1 on each of 3 questions on the Asthma Evaluation Questionnaire; eSupplement at http://www.jama.com). Patients whose lung function was less than 70% of predicted or had quantitatively greater symptom burden were allocated to the Tiotropium Bromide as an Alternative to Increased Inhaled Glucocorticoid in Patients Inadequately Controlled on a Lower Dose of Inhaled Corticosteroid (TALC) trial, which was a concurrently recruited Asthma Clinical Research Network trial.14
aDetails for those screened but ineligible were not collected.
bDropouts were included as censored observations.