Summary
Background
Fibrosis or inflammation of the bronchioles is a well-known manifestation of connective tissue disease (CTD). However, the natural history of CTD-related bronchiolitis is largely unknown.
Methods
We analyzed consecutive patients evaluated at National Jewish Health (Denver, CO) from 1998 to 2008 with CTD and surgical lung biopsy-confirmed bronchiolitis. Linear mixed effects models were used to estimate the longitudinal postbronchodilator FEV1 %predicted (%pred) course and differences between subjects with or without constrictive bronchiolitis (CB).
Results
Of 28 subjects with a mean age of 53 ± 9 years, fourteen (50%) had CB. The most common CTD diagnosis was rheumatoid arthritis (n = 14; 50%). There were no significant differences in demographics, smoking status, underlying CTD diagnoses, 6-min walk distance, dyspnea score or drug therapy between subjects with CB and those with cellular bronchiolitis. Three subjects with CB (11%) and four with cellular bronchiolitis (14%) died. Compared with subjects with CB, those with cellular bronchiolitis had higher mean FEV1 %pred at all times. There were no significant differences in FEV1 %pred slope within- or between-groups (CB vs. cellular bronchiolitis) preceding surgical lung biopsy or afterward.
Conclusion
Subjects with CTD-related CB had lower FEV1 %pred values than those with CTD-related cellular bronchiolitis at all time points, but FEV1 %pred remained stable over time in both groups regardless of therapy received.
Keywords: Pulmonary function testing, Autoimmune disease, Obliterative bronchiolitis
Introduction
Fibroinflammatory small airways involvement is a clinically important and frequent lung manifestation of connective tissue disease (CTD). Immunomodulatory therapies are often used in attempts to halt the progressive fibrous luminal narrowing of constrictive bronchiolitis (CB) or the transmural inflammation of cellular or follicular bronchiolitis in patients with connective tissue disease- (CTD-) related bronchiolitis. Pulmonary function tests (PFTs) are an essential part of standard monitoring in patients with CTD-related bronchiolitis; however, little is known about the natural history (or the effects of immunomodulatory therapy on lung function over time) of bronchiolar disease in these patients.1–7 Data on longitudinal trends in lung function is essential for patient management decisions and for designing therapeutic trials.
The objectives of this study were to examine the longitudinal course of postbronchodilator FEV1 %predicted (%pred) in a cohort of well-characterized patients with CTD-related bronchiolitis and to explore whether the course of FEV1 differed between patients with CB and those with cellular CTD-related bronchiolitis.
Methods
Study design and patients
We searched the National Jewish Health Interstitial Lung Disease Program Research database and identified consecutive adult patients with CTD and clinical–radiologic–pathologic evidence of bronchiolitis (in the absence of interstitial lung disease) who were evaluated in our program between January 1, 1998 and January 1, 2008. The database of the Interstitial Lung Disease Program at our institution includes more than 4000 patients who are registered and followed up prospectively. As part of their comprehensive evaluation, all patients underwent standardized clinical evaluation that included serologic testing, thoracic high-resolution computed tomography (HRCT) scan, and PFTs. We reviewed the complete medical records of included subjects and abstracted data pertaining to the following: demographics, smoking history, CTD diagnosis, postbronchodilator percent predicted PFTs, 6-min walk, dyspnea score,8 imaging, histopathology and treatment. A pulmonologist with expertise in bronchiolar diseases and a board-certified rheumatologist evaluated every subject. The National Jewish Health Institutional Review Board approved this study (protocol HS# 1603). All the patients on the research database had previously provided informed consent.
Inclusion criteria
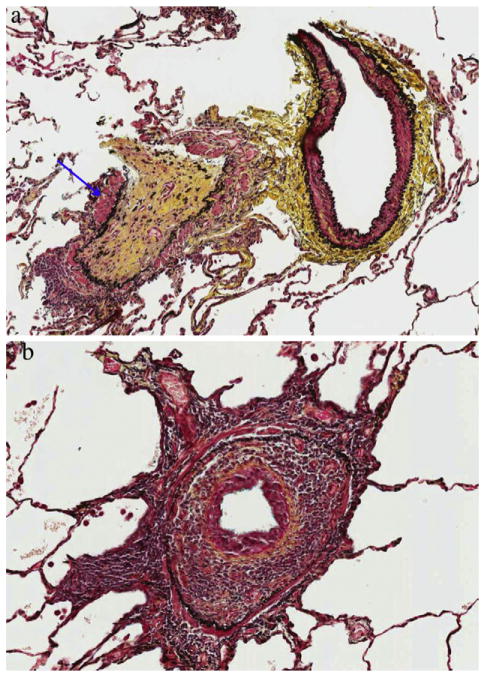
All patients met American College of Rheumatology criteria for a specific CTD. Diagnostic criteria for bronchiolitis included the following: (1) presence of compatible clinical features such as dyspnea, cough, chest tightness, and wheezing or inspiratory squeaks on auscultation; (2) abnormal PFT such as an obstructive pattern according to the guidelines of the American Thoracic Society9; (3) compatible HRCT (including expiratory images) features including one or more of the following findings: centrilobular nodules, tree-in-bud pattern, mosaic attenuation, and air trapping; (4) absence of an alternative diagnosis that could account for the pathologic or HRCT findings (e.g., interstitial lung disease such as hypersensitivity pneumonitis, or bronchiolitis obliterans organizing pneumonia, neuroendocrine cell hyperplasia or infection based on bacterial, fungal and mycobacterial smears and cultures); (5) Surgical lung biopsy (SLBx) specimens showing primarily bronchiolitis. CB was defined histologically by concentric luminal narrowing of the membranous and respiratory bronchioles by submucosal and peribronchiolar fibrosis without intraluminal granulation tissue or polyps (Fig. 1(a)).10,11 For the purpose of this study, non-CB was defined by pathologic patterns of bronchiolitis that were not CB, including either a chronic lymphoplasmacytic cell infiltrate in the walls of small airway (cellular bronchiolitis-Fig. 1(b)) or the presence of hyperplastic lymphoid follicles with reactive germinal centers distributed along bronchioles (follicular bronchiolitis).10,11
Figure 1.
a. Constrictive bronchiolitis: bronchovascular bundle showing complete airway luminal obliteration with replacement of the bronchiole mucosa by a relatively acellular scar (arrow). Pentachrome stain ×20 magnification. b. Cellular bronchiolitis: peribronchiolar mononuclear cellular infiltrate showing concentric luminal narrowing of the airway. Pentachrome 40× magnification.
Thoracoscopic biopsies were performed, with biopsy sites selected by the surgeon (≥2). An expert pulmonary pathologist, blinded to all clinical information, had previously reviewed all biopsies. On surgical lung biopsy, all specimens demonstrated concordant histopathologic findings. All patients with a bronchiolitis pattern in SLBx specimens also had a pattern of bronchiolitis on HRCT.
Statistical analysis
Descriptive statistics were generated for baseline characteristics. Wilcoxon, Student t-tests, chi-square test and Fisher exact tests were used for univariate comparisons as appropriate. The date of SLBx was accepted as the date of diagnosis. Survival period was calculated starting from the date of diagnosis until death. Vital status was ascertained on September 30, 2012 via query of the Social Security Death Index (SSDI). Subjects not appearing in the SSDI were censored on that date. Given the retrospective design, we needed to account for the lack of uniformity in the timing of spirometric assessments among subjects (e.g., some had spirometry two months after diagnosis, others had them three months after). Instead of deleting subjects case-wise because of this heterogeneity in data collection, or using biased imputation methods, we chose to take advantage of the benefits of mixed-effects models (SAS Proc Mixed) to assess spirometric assessments over time. We used this analytic method to model spirometric assessments as a function of time before and after SLBx, while adjusting for age, gender. Time was measured in weeks relative to the date of SLBx (time 0), and time points of interest were −26 weeks, time 0, +52, +104 and +156 weeks. All statistical testing was performed at the conventional 2-tailed alpha level of 0.05. Statistical software packages (JMP, version 9 and SAS version 9.2, SAS Institute Inc., Cary, NC) were used for data analyses.
Results
Patient characteristics
Of a total of 43 patients identified, 15 were excluded (eight with primary interstitial lung diseases, two with primary immunodeficiencies, four did not have a definite CTD diagnosis and one had chronic mycobacterial infection). The final cohort consisted of 28 subjects with a mean age of 53 ± 9 years. Fourteen (50%) had CB. All patients had a HRCT pattern consistent with bronchiolitis and without opacities suggesting interstitial lung disease. The most common underlying CTD diagnoses were rheumatoid arthritis (n = 14; 50%), primary Sjögren’s syndrome (n = 7; 25%) and undifferentiated connective tissue disease (n = 5, 18%). Table 1 displays the clinical characteristics of subjects stratified on the presence or absence of CB. At the time of diagnosis, there were no significant differences in demographics, smoking status, underlying CTD diagnosis, 6-min walk distance or therapy between subjects with or without CB.
Table 1.
Baseline characteristic among patients with connective tissue diseases with and without constrictive bronchiolitis.
| Characteristics | All patients (N = 28) | Histopathologic pattern+
|
P value | |
|---|---|---|---|---|
| CB (n = 14) | Non-CB (n = 14) | |||
| Median follow-up, years | 5.5 (3.9–8.2) | 5.5 (4.3–7.7) | 5.2 (3.5–8.3) | 0.67 |
| Mortality rate no. (%) | 7 (25) | 3 (21) | 4 (28) | 0.66 |
| Age (years) | 53 ± 9 | 54 ± 6 | 51 ± 11 | 0.44 |
| Female sex no. (%) | 21 (75) | 10 (71) | 11 (78) | 0.66 |
| Body-mass index | 28 ± 6 | 28 ± 6 | 27 ± 7 | 0.40 |
| Smoking status | ||||
| Previous smoker no. (%) | 6 (21) | 3 (21) | 3 (21) | 1.00 |
| Smoking history (pack-year) | 22 ± 15 | 21 ± 16 | 24 ± 17 | 0.85 |
| Phenotype no. (%) | ||||
| Rheumatoid arthritisa | 14 (50) | 8 (57) | 6 (42) | |
| Primary Sjögren’s syndrome | 7 (25) | 1 (7) | 6 (42) | 0.11 |
| Undifferentiated connective tissue disease | 5 (18) | 3 (21) | 2 (14) | |
| Otherb | 2 (7) | 2 (14) | 0 | |
| Pulmonary function tests, %predictedc | ||||
| TLC | 97 ± 18 | 94 ± 17 | 100 ± 19 | 0.40 |
| RV | 181 ± 70 | 172 ± 64 | 190 ± 78 | 0.53 |
| FVC | 60 ± 18 | 55 ± 15 | 65 ± 20 | 0.23 |
| FEV1 | 49 ± 23 | 43 ± 18 | 55 ± 27 | 0.18 |
| FVC/FEV1 ratio | 74 ± 20 | 73 ± 20 | 76 ± 21 | 0.79 |
| DLCO | 66 ± 18 | 64 ± 17 | 69 ± 20 | 0.49 |
| 6-Minute walk | ||||
| Distance (feet) | 1110 (926–1596) | 1120 (967–1579) | 976 (570–1650) | 0.48 |
| Nadir SpO2 | 91 (89–94) | 91 (89–94) | 92 (88–93) | 0.96 |
| Supplemental O2 flow rate | 0.5 (0–3) | 0 (0–2) | 2 (0–5) | 0.26 |
| Heart rate recoveryd | 20 (16–23) | 17 (15–22) | 22 (20–23) | 0.16 |
| Dyspnea scoree | 9 ± 5 | 9 ± 7 | 9 ± 6 | 0.74 |
| Bronchiolitis medications no. (%) | ||||
| Prednisone | 28 (100) | 14 (100) | 14 (100) | 0.90 |
| Azathioprine | 4 (14) | 3 (21) | 1 (7) | 0.27 |
| Mycophenolate mofetil | 13 (46) | 8 (57) | 5 (36) | 0.25 |
| Cyclophosphamide | 10 (35) | 7 (50) | 3 (21) | 0.07 |
| Macrolide | 9 (32) | 4 (28) | 5 (35) | 0.68 |
| Treatment period (months) | ||||
| Prednisone | 13.5 (9–105) | 13.5 (9–54) | 24 (12–129) | 0.41 |
| Azathioprine | 14 (3–24) | 13.5 (3–24) | 14 (13–14) | 1.00 |
| Mycophenolate mofetil | 19.5 (11.3–57) | 36 (12–60) | 12 (7–43) | 0.29 |
| Cyclophosphamide | 11.5 (5.8–15) | 12 (6–24) | 11 (5–12) | 0.49 |
| Macrolide | 12 (9.3–20.3) | 23 (12–48) | 10 (7–12) | 0.05 |
Data are expressed as mean ± standard deviation, number (%) or median (25–75% quartiles). CB, constrictive bronchiolitis.
Two patients with rheumatoid arthritis had secondary Sjögren (one with constrictive bronchiolitis and one with non-constrictive bronchiolitis).
Other: scleroderma, microscopic polyangiitis.
Mean percent of predicted: TLC, total lung capacity; RV, residual volume; FVC, forced vital capacity; FEV1, force expiratory volume in one second; Dlco, diffusing capacity of the lung for carbon monoxide. No patient had restriction as measured by TLC. Six subjects (2 constrictive bronchiolitis, 4 non-constrictive bronchiolitis) had an FEV1/FVC ratio ≥70, elevated RV/TLC ratios and mildly reduced FEV1at the time of surgical lung biopsy diagnosis.
Heart rate recovery: defined as the difference between a subject’s heart rate at the 6th minute of the 6-min walk test and at 1 min after completion of the 6-min walk test.
ATS dyspnea score: higher score means more deterioration in severity of dyspnea (Reference 7).
Bronchiolitis pattern on surgical lung biopsy.
Systemic corticosteroids were recommended in all patients (mean dose 23 ± 14 mg). In patients who did not respond to corticosteroids according to clinical measures (e.g., symptoms, gas exchange, physiology), cytotoxic therapy (i.e. azathioprine, mycophenolate mofetil, cyclophosphamide) by means of a standardized treatment and monitoring protocols was recommended.12 Before SLBx diagnosis of bronchiolitis in those with rheumatoid arthritis, four had received methotrexate, three had received hydroxychloroquine, one had received sulfasalazine and one was treated with leflunamide. None received gold salts or d-penicillamine.
Rate of change in FEV1
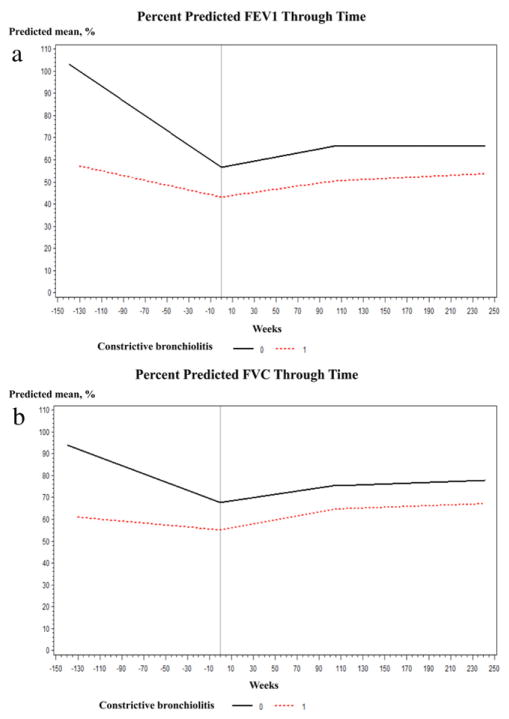
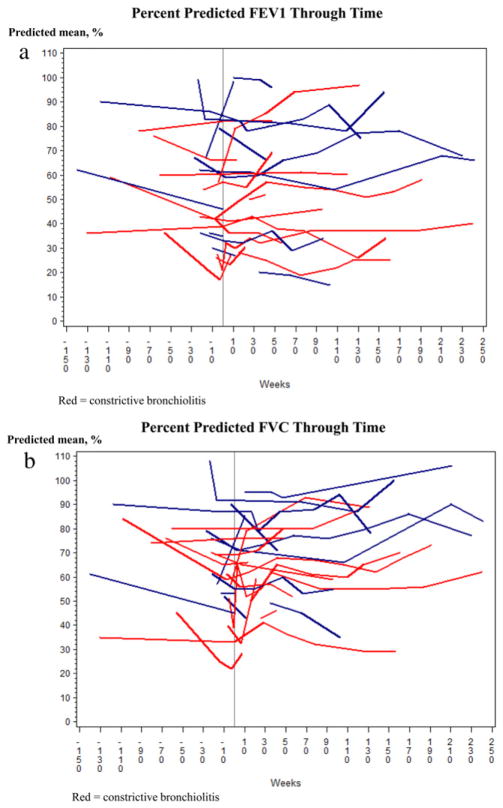
Compared with patients with CB, those with non-CB had higher predicted mean FEV1 %pred and FVC %pred at all times points during the study period (Fig. 2(a) and (b)) especially at 26 weeks before diagnosis, at diagnosis and at 52 weeks (Table 2). There were no significant differences either within- or between-group estimates for the FEV1 %pred and FVC %pred change before and after diagnosis (Table 3). We found no relationship between the course of FEV1 and the number of FEV1 measurements (data not shown, p = 0.69), suggesting that subjects with the greatest number of observations did not bias model estimates. Spaghetti plots of measured FEV1% and FVC% are displayed in Fig. 3(a) and (b).
Figure 2.
a. Mixed-effect model plot of derives estimates for post-bronchodilator forced expiratory volume in one second percent predicted over time stratified by the presence or absence of constrictive bronchiolitis. b. Mixed-effect model plot of derives estimates for post-bronchodilator forced vital capacity percent predicted over time stratified by the presence or absence of constrictive bronchiolitis.
Table 2.
Age and gender adjusted within- and between-group estimates for postbronchodilator forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) at time points before and after diagnosis (N = 28).
| 26 weeks before diagnosis
|
At diagnosis
|
At 52 weeks
|
At 104 weeks
|
At 156 weeks
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CB | Non-CB | CB | Non-CB | CB | Non-CB | CB | Non-CB | CB | Non-CB | |
| FEV1% | 46.0 ± 4.4 Difference |
65.3 ± 4.1 | 43.2 ± 3.4 | 56.6 ± 3.7 | 46.7 ± 5.8 | 61.4 ± 4.3 | 50.3 ± 8.4 | 66.2 ± 8.5 | 51.5 ± 11.9 | 66.2 ± 11.3 |
| −19.2 ± 5.3 | p < 0.01 | −13.4 ± 5.1 | p < 0.01 | −14.6 ± 6.1 | p = 0.02 | −15.9 ± 12.0 | p = 0.18 | −14.6 ± 16.4 | p = 0.37 | |
| FVC% | 56.2 ± 2.8 Difference |
72.5 ± 3.3 | 55.1 ± 2.9 | 67.6 ± 3.2 | 59.8 ± 3.1 | 71.5 ± 3.2 | 64.5 ± 6.2 | 75.3 ± 6.2 | 65.5 ± 8.3 | 76.2 ± 7.5 |
| −16.2 ± 8.3 | p < 0.01 | −12.5 ± 4.4 | p < 0.01 | −11.6 ± 4.4 | p = 0.01 | −10.8 ± 8.8 | p = 0.22 | −10.7 ± 11.2 | p = 0.34 | |
CB, constrictive bronchiolitis.
Table 3.
Age and gender adjusted within- and between-group estimates for postbronchodilator forced expiratory volume in one second (FEV1) and forced vital capacity change before and after diagnosis (N = 28).
| Change from 26 weeks to diagnosis
|
Change from diagnosis to 52 weeks
|
Change from diagnosis to 104 weeks
|
Change from diagnosis to 156 weeks
|
|||||
|---|---|---|---|---|---|---|---|---|
| CB | Non-CB | CB | Non-CB | CB | Non-CB | CB | Non-CB | |
| FEV1% | 2.7 ± 2.8 Difference |
8.6 ± 3.6 | 3.5 ± 4.8 | 4.8 ± 4.9 | 7.1 ± 9.8 | 9.6 ± 9.9 | 8.3 ± 12.5 | 9.5 ± 12.4 |
| −5.8 ± 4.6 | p = 0.21 | −1.2 ± 6.9 | p = 0.86 | −2.5 ± 13.9 | p = 0.85 | −1.2 ± 17.5 | p = 0.94 | |
| FVC% | 1.2 ± 2.2 Difference |
4.9 ± 2.8 | 4.7 ± 3.7 | 3.8 ± 3.7 | 9.4 ± 7.5 | 7.7 ± 7.5 | 10.4 ± 8.9 | 8.7 ± 8.6 |
| −3.7 ± 3.6 | p = 0.31 | 0.85 ± 5.3 | p = 0.87 | 1.7 ± 10.7 | p = 0.87 | 1.8 ± 12.4 | p = 0.88 | |
CB, constrictive bronchiolitis.
Figure 3.
a. Spaghetti plot for forced expiratory volume in one second percent predicted for subjects with and without constrictive bronchiolitis; each subject represents a single subject. b. Spaghetti plot for forced vital capacity percent predicted for subjects with and without constrictive bronchiolitis; each subject represents a single subject.
Mortality
Three patients with CB (23%) and four with non-CB (33%) died during a median follow up of 5.5 years. Male gender was associated with increased mortality (5 of 7 male patients, (71%) vs. 2 of 21 female patients, (9%)). Of the seven deaths, five were due to progressive respiratory failure. The cause of death was unknown in the remaining patients.
Discussion
To our knowledge, this is the first study to assess the longitudinal behavior of FEV1 in patients with CTD-related, SLBx-proven bronchiolitis. We observed that, although subjects with CB had significantly lower FEV1 values than subjects with non-CB, the rate of change of FEV1 did not vary significantly in either subgroup for at least three years after SLBx.
Our PFT findings in patients CB at study entry are consistent with that reported in patients with rheumatoid arthritis-related CB.13 CB is a leading cause of morbidity and late mortality after lung transplantation.14 Although non-transplant CB is regarded as a progressive disease,13,15–17 we did not observe a significant decline in FEV1 over time in the subgroup with CB. This suggests that, in patients with CTD-related CB, FEV1 may plateau for prolonged periods of time, and the natural history of CTD-related bronchiolitis (CB or non-CB) may progress in a stair-step pattern.
Our findings challenge the concept that unrelenting, progressive loss of lung function is inevitable in CB. If replicated in subsequent studies, this promising news could influence patient counseling and disease management by avoiding unnecessary treatment in inherently stable disease. Similarly, and to our surprise, we did not observe a significant increase in FEV1 over time in the subgroup with non-CB. We had expected to see improvements in FEV1 in this subgroup after treatment.
There was no difference between genders in FEV1 slopes before or after surgical lung biopsy. Nor was baseline FEV1 associated with changes over time in FEV1 (data not shown): the estimated FEV1 values over time in subjects with lower FEV1 values at baseline (e.g., 50% predicted or less) remained low over the course of the study, but the FEV1 slope was no different from subjects with higher (e.g., >50% predicted) baseline FEV1 values.
An additional important finding of our study is the impact of gender on survival of CTD-related bronchiolitis. In our study, male gender was found to be associated with increased mortality and was the only predictor at diagnosis in univariate time-to-death analysis (hazard ratio 7.5, 95% CI 1.6–23.1, p = 0.01). Another important, practical observation reflects the finding that subjects with CB had survival rates similar to subjects with non-CB. The implication is that airway histology alone does not dictate prognosis and there are likely multiple factors that do.
Our study has limitations. First, the small sample size leaves open the possibility that the estimates our models yielded do not accurately reflect FEV1 course in the greater population of patients with CTD-related bronchiolitis. However, because each subject underwent SLBx (study inclusion criteria) and thorough rheumatological evaluation, we can be highly confident in the accuracy of the reported airway and CTD phenotypes. This is a sample with well-defined disease; and we believe this study is a promising first step in advancing understanding of CTD-related bronchiolitis. Second, subjects had differing amounts of analyzable FEV1 data, and the timing of assessments within any one subject may not have occurred at consistent intervals over time. Mixed-effects models are perfectly suited for analyzing this type of data.18 These models accommodate the inherent irregularities (e.g., differences between subjects in the number of assessments and the inconsistent timing of assessments within subjects) in retrospective data; they allow for missing data, thus obviating the need for case-wise deletion; they handle time-varying covariates and parameterize the within-subject correlation naturally occurring in repeated measures data. Third, we had no control of when or whether drug therapy was initiated or what agents were used. In general, the approach to therapy was similar between subjects–subjects with more severe airflow limitation were given a trial of immunomodulatory therapy. Consistent with this, our analysis of FEV1 over time with the sample stratified on whether or not cyclophosphamide (CYC) was used at any time during the course of disease (data not shown) revealed that subjects who received CYC had lower FEV1 at all time points than those who did not receive CYC. There was no difference in FEV1 slope (either before or after surgical lung biopsy) between the two groups. Because of the lack of systematic implementation of therapy, we are unable to confidently make any inferences about the effect of therapy on the course of FEV1 in patients with CTD-related bronchiolitis. Clearly, rationalization and efficacy of treatment cannot be evaluated without carefully constructed randomized therapeutic trials. Finally, the diagnosis and management of CTD-related bronchiolitis in our subjects was carried out at a specialized referral center, and our results may not extend to patients in the community.
In conclusion, in this highly selected, well-characterized sample of subjects with treated CTD-bronchiolitis, postbronchodilator FEV1 values were lower over the course of the study for the subgroup with CTD-related CB than for the subgroup with CTD-related non-CB. However, there was no difference in the FEV1 slope between subgroups. Additional investigations in larger, but equally well-defined samples will build on the results of this study and improve understanding of CTD-related bronchiolitis.
Footnotes
Financial disclosure
The authors do not have any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
Conflict of interest
The authors do not have any conflict of interest.
References
- 1.Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with rheumatoid arthritis. Mod Rheumatol. Apr;21(2):164–173. doi: 10.1007/s10165-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borie R, Schneider S, Debray MP, et al. Severe chronic bronchiolitis as the presenting feature of primary Sjogren’s syndrome. Respir Med. Jan;105(1):130–136. doi: 10.1016/j.rmed.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Wells AU, du Bois RM. Bronchiolitis in association with connective tissue disorders. Clin Chest Med. 1993 Dec;14(4):655–66. [PubMed] [Google Scholar]
- 4.Perez T, Remy-Jardin M, Cortet B. Airways involvement in rheumatoid arthritis: clinical, functional, and HRCT findings. Am J Respir Crit Care Med. 1998 May;157(5 Pt 1):1658–65. doi: 10.1164/ajrccm.157.5.9710018. [DOI] [PubMed] [Google Scholar]
- 5.Hakala M, Paakko P, Sutinen S, Huhti E, Koivisto O, Tarkka M. Association of bronchiolitis with connective tissue disorders. Ann Rheum Dis. 1986 Aug;45(8):656–62. doi: 10.1136/ard.45.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuld JP, Johnson MK, Cotton MM, et al. A longitudinal study of lung function in nonsmoking patients with rheumatoid arthritis. Chest. 2003 Oct;124(4):1224–31. doi: 10.1378/chest.124.4.1224. [DOI] [PubMed] [Google Scholar]
- 7.Avnon LS, Manzur F, Bolotin A, et al. Pulmonary functions testing in patients with rheumatoid arthritis. Isr Med Assoc J. 2009 Feb;11(2):83–7. [PubMed] [Google Scholar]
- 8.Watters LC, King TE, Schwarz MI, Waldron JA, Stanford RE, Cherniack RM. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1986 Jan;133(1):97–103. doi: 10.1164/arrd.1986.133.1.97. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005 Nov;26(5):948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 10.Pipavath SJ, Lynch DA, Cool C, Brown KK, Newell JD. Radiologic and pathologic features of bronchiolitis. AJR Am J Roentgenol. 2005 Aug;185(2):354–63. doi: 10.2214/ajr.185.2.01850354. [DOI] [PubMed] [Google Scholar]
- 11.Allen TC. Pathology of small airways disease. Arch Pathol Lab Med. 2010 May;134(5):702–18. doi: 10.5858/134.5.702. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Meyer KC, Nathanson I, et al. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Nov;142(5):e1S–111S. doi: 10.1378/chest.12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devouassoux G, Cottin V, Liote H, et al. Characterisation of severe obliterative bronchiolitis in rheumatoid arthritis. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2009 May;33(5):1053–61. doi: 10.1183/09031936.00091608. [DOI] [PubMed] [Google Scholar]
- 14.Christie JD, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: twenty-eighth adult lung and heart-lung transplant Report-2011. J Heart Lung Transplant. 2011 Oct;30(10):1104–22. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 15.King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011 Jul 21;365(3):222–30. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003 Dec 1;168(11):1277–92. doi: 10.1164/rccm.200301-053SO. [DOI] [PubMed] [Google Scholar]
- 17.Geddes DM, Corrin B, Brewerton DA, Davies RJ, Turner-Warwick M. Progressive airway obliteration in adults and its association with rheumatoid disease. Q J Med. 1977 Oct;46(184):427–44. [PubMed] [Google Scholar]
- 18.Mehrotra DV. A comparison of generalized linear mixed model procedures with estimating equations for variance and covariance parameter estimation in longitudinal studies and group randomized trials. Stat Med. 2002 Dec 15;21(23):3745–7. doi: 10.1002/sim.1245. author reply 3747–3748. [DOI] [PubMed] [Google Scholar]