Figure 2.
Programming single-species' populations via engineered cell-cell communication.
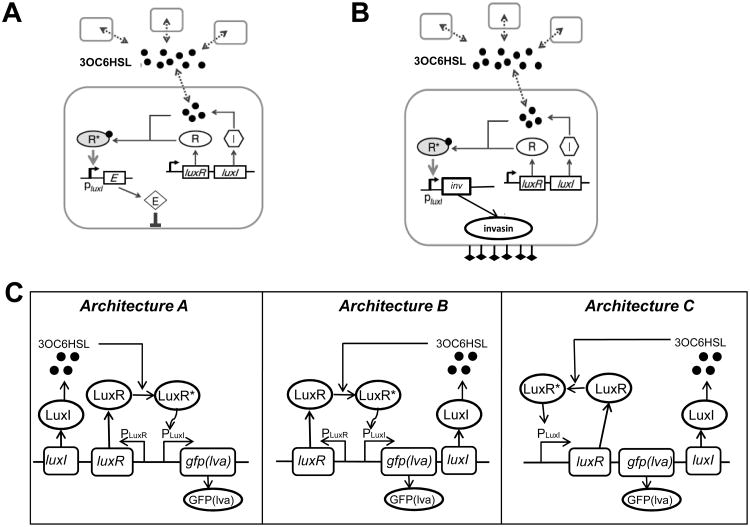
(A) A population-control circuit which modulates population density of a single species. The circuit uses the LuxR/LuxI QS circuit, wherein both LuxR and LuxI genes are under the control of the Plac promoter, inducible by IPTG. LuxI produces 3OC6HSL, which can bind and activate LuxR at a sufficiently high concentration. Activated LuxR-3OC6HSL complex (R*) in turn binds to the PluxI promoter to induce expression of a killer gene, ccdB. High expression of ccdB leads to cell killing, thus reducing population density. Figure adapted from You et al. [41].
(B) Synthetic QS circuit to control bacterial invasion of malignant cancer cells. Employing a similar network structure as (A), the killer gene (LacZα-ccdB) in (A) is replaced by an invasion gene inv. Thereby, the invasion of mammalian cells by the programmed bacteria is under the control of QS. Figure adapted from Anderson et al. [42].
(C) Three network architectures for QS gene circuits synthesized by circuit reshuffling. Architecture A: LuxI and LuxR are constitutively expressed, GFP(lva) is under the control of the PluxI promoter, which can be activated by the LuxR-autoinducer complex (LuxR*). Architecture B: LuxR is constitutively expressed; LuxI synthesizes 3OC6HSL and autoactivates expression of itself upon binding with LuxR, forming a positive-feedback loop. Architecture C: Expression of LuxR and LuxI are all under the control of the positive-feedback loop consisting of LuxR-autoinducer and the PluxI promoter. Figure adapted from Haseltine and Arnold [43].
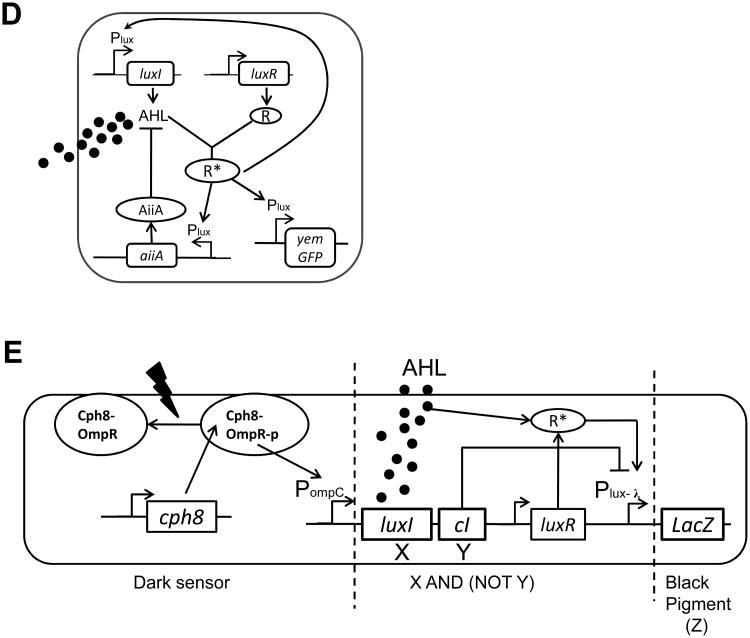
(D) The genetic diagram of a synchronized genetic oscillator. The Plux promoter induces the production of three genes (LuxI, aiiA and yemGFP). The activated LuxR-AHL complex (R*) binds the Plux promoter and activates gene expression. AiiA is an effective protease that catalyzes the cleavage of AHL. The overall circuit functions as coupled negative and positive feedback loops, wherein AHL activates synthesis of itself (positive feedback), and induces AiiA expression, which in turn represses AHL's accumulation by catalyzing AHL's degradation (negative feedback). Figure adapted from Danino et al. [44].
(E) The genetic circuit dictating an edge detection algorithm. The genetic cascade includes three sequential parts: First, the dark sensor part includes a light-sensitive kinase protein Cph8. In the presence of red light, the Cph8 kinase activity is inhibited, precluding the transfer of a phosphoryl group to the response regulator OmpR and subsequent transcriptional activation of the OmpC promoter (PompC). The dark sensor therefore functions as a NOT logic gate. Second, in the X and (NOT Y) logic gate, the PompC promoter drives the synthesis of LuxI and CI. LuxI produces a quorum-sensing signal AHL. CI inhibits the Plux- λ promoter. LuxR-AHL complex (R*) activates the Plux- λ promoter. Therefore, Plux- λ functions as an X and (NOT Y) gate. Lastly, the Plux- λ promoter drives the synthesis of LacZ, which produces β-galactosidase, an enzyme that subsequently cleaves a substrate in the media to produce black pigment. Figure adapted from Tabor et al. [45].
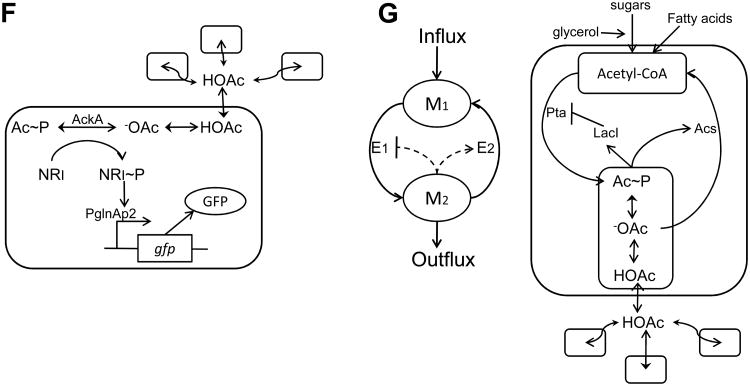
(F) Artificial cell-cell communication using a metabolite signal. Amino acid metabolism results in a metabolite, acetate, as a byproduct. Acetic acid (HOAc), diffuses freely across the cell membrane, playing the role of an artificial cell-cell communication signal. In the cytoplasm, -OAc can be phosphorylated to acetyl phosphate (Ac∼P), which can transfer its phosphate group to NR1. Upon phosphorylation, NR1∼P becomes a transcriptional regulator of the glnAp2 promoter which activates expression of GFP. Therefore, the GFP level reflects the acetate concentration, which in turn is correlated with cell density. Figure adapted from Butler et al. [38].
(G) Metabolator, a synthetic gene-metabolic oscillator that integrates transcriptional regulation with metabolism. The left panel shows the conceptual diagram of the circuit design in which the two metabolite pools (M1 and M2) are controlled by two enzymes (E1 and E2). The right panel illustrates the biological realization of the conceptual design. Acetyl-CoA is converted to acetyl phosphate (Ac∼P) by phosphate acetyltransferase (Pta), which converts to acetate (-OAc) by acetate kinase (Ack). Meanwhile, the enzyme acetyl-CoA synthetase (Acs) is induced in the presence of acetate (-OAc). When Ac∼P reaches a critical concentration, it represses the expression of Pta. Figure adapted from Fung et al. [46].