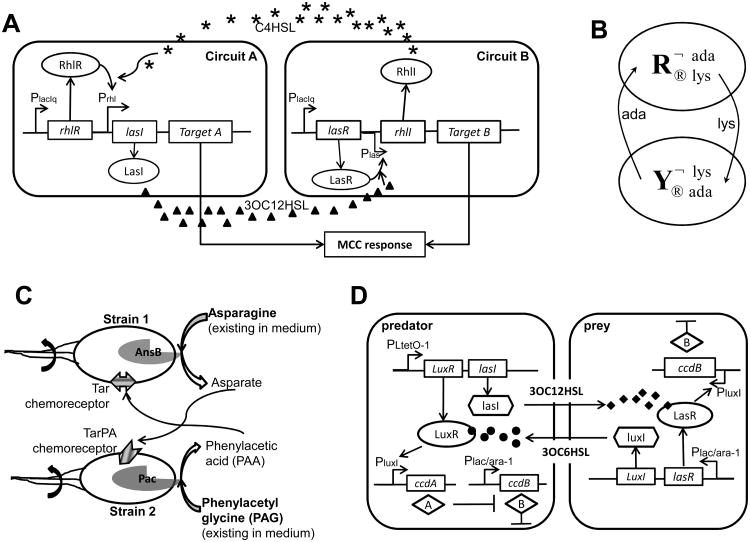
Figure 4.
Engineering two-way cell-cell communication.
(A) A microbial consensus consortium (MCC) circuit. It implements two-way cell-cell communication using two QS modules, LasR/LasI and RhlR/RhlI, to achieve a consensus response. In Circuit A, LasI produces 3OC12HSL, which diffuses into cells containing Circuit B and binds LasR, thereby inducing expression of RhlI. RhlI in Circuit B synthesizes C4HSL, which diffuses into cells containing Circuit A and binds RhlR to activate LasI expression, closing the positive feedback loop. Figure adapted from Brenner et al. [31].
(B) A synthetic obligatory cooperative system achieved by exchanging metabolites in a two-way communication system. This system consists of two auxotrophic yeast strains that depend on each other for survival. One strain, is unable to synthesize adenine but overproduces lysine. In contrast, another strain, is unable to synthesize lysine but overproduces adenine. Figure adapted from Shou et al. [40].
(C) An engineered cooperative microbial consortium, in which only the mixture of the two bacteria can chemotax toward the combined gradient of asparagine and phenylacetyl glycine (PAG). One strain synthesizes penicillin acylase (Pac), which enzymatically hydrolyzes phenylacetyl glycine (PAG) to produce phenylacetic acid (PAA), a chemoattractant to E. coli cells with Tar chemoreceptors. The other strain expresses AnsB gene, which encodes Asparaginase. Asparaginase enzymatically converts Asparagine to Aspartate, functioning as a ligand (or chemoattractant) to E. coli cells with TarPA chemoreceptors. This engineered mutualistic consortium is not able to chemotax toward a gradient consisting of only one of the two compounds (Asparagine and PAA). Figure adapted from Goldberg et al. [55].
(D) Schematic of the gene circuit in a synthetic predator-prey ecosystem. The system consists of two types of engineered E. coli. Each controls the other's survival and death via two QS circuits, LuxR/LuxI from V. fisheri and LasR/LasI from P. aeruginosa. The predator synthesizes 3OC12HSL, which diffuses into the prey and binds to LasR to induce expression of the ccdB killer protein, thereby killing the prey. On the other hand, the prey synthesizes 3OC6HSL, which diffuses into the predator and binds to LuxR to induce expression of the ccdA antidote protein. This protein neutralizes the toxicity of the ccdB killer protein, thereby rescuing the predator. Figures adapted from Balagadde et al. [32].