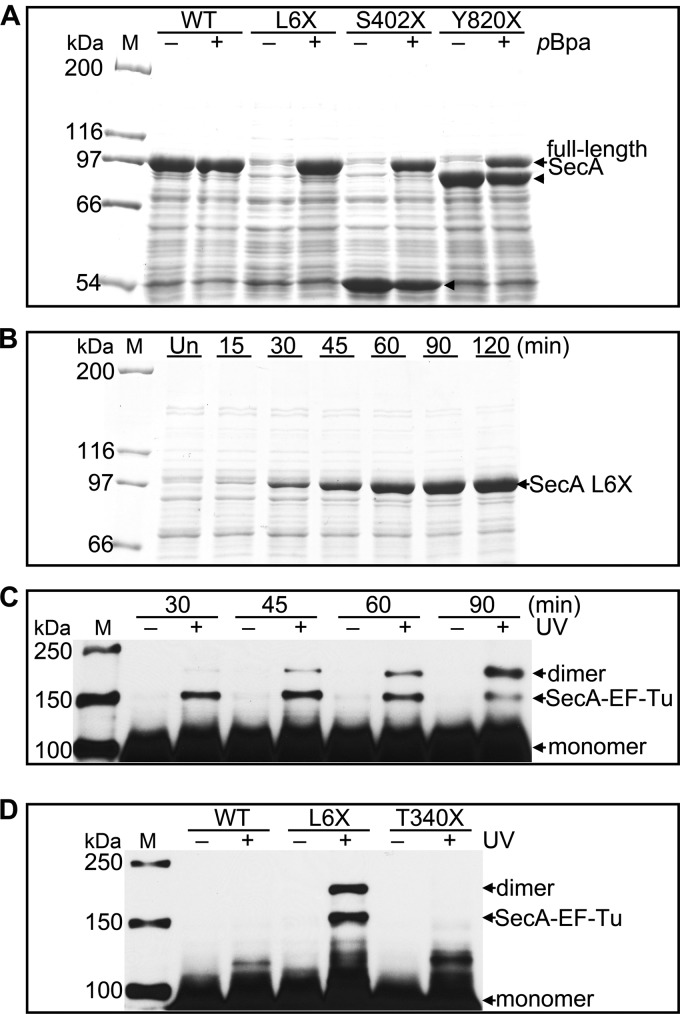
Fig 2.
Expression, identification, and specificity of SecA site-specific in vivo photo-cross-linking. (A) SDS-PAGE analysis of the induction of wild-type SecA (WT) and SecA L6X, S402X, and Y820X in the absence (−) and in the presence (+) of pBpa. Full-length SecA is produced only in the presence of pBpa. The arrowheads indicate SecA fragments that likely prematurely terminated at the amber codon. Their molecular masses are consistent with the location of the amber codon used for pBpa incorporation. The corresponding fragment from SecA L6X is so small that it cannot be detected by 7.5% SDS-PAGE. (B) SDS-PAGE analysis of SecA L6X expression at the indicated times after IPTG induction. Un, uninduced cells. (C) Western blot analysis of lysates from either untreated (−) or UV-irradiated (+) cells expressing SecA L6X harvested at the indicated times following induction. SecA dimer and SecA–EF-Tu, confirmed by LC-MS/MS, and monomeric SecA are indicated. (D) Comparison of the UV cross-linking pattern of SecA L6X and those of wild-type SecA (WT) and SecA T340X. Samples for panels A and B were from whole-cell lysates. Samples for panels C and D were from the soluble fraction of cell lysates. A monoclonal anti-His antibody was used for immunoblot analysis as described in Materials and Methods. Lanes M, protein molecular mass markers, with molecular masses shown on the left.