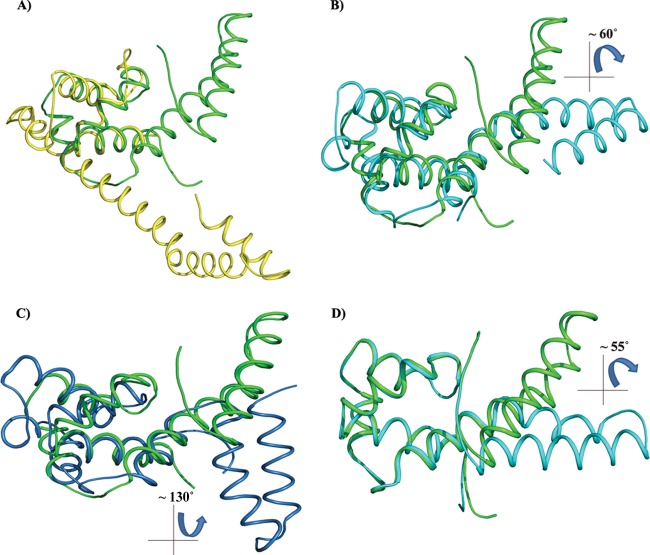
Fig 3.
Superposition of the HpDnaG-CTD crystal structure with the crystal structure of EcDnaG-CTD and BstDnaG-CTD (solution and crystal) structures. Because of its flexibility, the helical hairpin region (HHR) may have different orientations when in a free or bounded state. The figure shows how the globe region (GR) of H. pylori, when superposed, gives insight into the differences in the shapes and orientations of HHR. The EcDnaG-CTD HHR is small and not flexible, but BstDnaG-CTD HHR is flexible. (A) Superposition of HpDnaG-CTD crystal structure (green) with EcDnaG-CTD (yellow). (B) Superposition of HpDnaG-CTD crystal structure (green) with BstDnaG-CTD crystal structure (cyan) shows the difference of about 60° in hairpin orientations. The angle is measured between Phe534, Arg522 of HpDnaG-CTD, and Arg574 of BstDnaG-CTD. (C) Superposition of HpDnaG-CTD crystal structure (green) with BstDnaG-CTD solution or NMR model (blue) highlights the flexibility of hairpin as seen by huge angular difference of about 130° in opposite side of helicase binding axis. (D) Green helical hairpin, the native HpDnaG-CTD crystal structure was rotated by about 55°. It is shown in cyan to model the bound state in the model (as shown in Fig. 4 in red). Owing to the flexibility of HHR, the large rotation of helical hairpin of HpDnaG-CTD was performed to model the complex with BstDnaB-BstDnaG-CTD (helicase-primase) complex as a template.