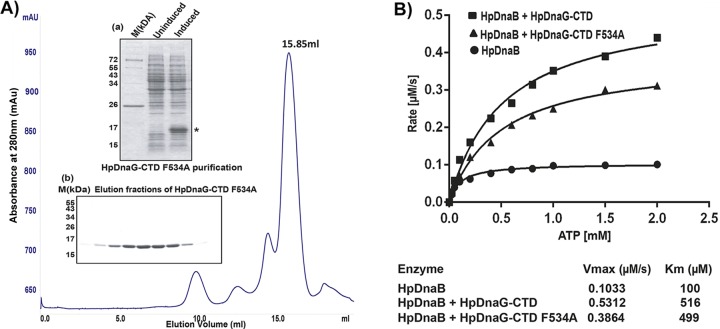
Fig 7.
Purification of HpDnaG-CTD F534A mutant and its effect on HpDnaB (wild type) ATPase activity. (A) Gel filtration chromatogram of HpDnaG-CTD F534A (mutant) obtained by passage through a Superdex-200 10/300 GL (GE Healthcare) column. Inset panel a shows the induction profile of HpDnaG-CTD F534A (indicated by an asterisk), along with marker (M), and inset panel b shows a Coomassie blue-stained gel corresponding to the peak fractions (15.85 ml) collected after gel filtration of HpDnaG-CTD F534A. (B) Stimulation of HpDnaB ATPase activity in the presence of HpDnaG-CTD or HpDnaG-CTD F534A mutant protein. ATPase assays were performed as described previously in Materials and Methods. ATP hydrolysis rates were calculated for the HpDnaB alone or in combination with HpDnaG-CTD or HpDnaG-CTD F534A mutant protein (each 1.3 μM). ATP hydrolysis rates were plotted against substrate concentration (ATP) as shown in figure. Alone HpDnaG-CTD (wild type or mutant protein) did not show any ATPase activity (data not shown) but both stimulated HpDnaB ATPase activity. However, the presence of HpDnaG-CTD F534A mutant protein showed overall decrease (∼30%) in ATP hydrolysis rate than HpDnaG-CTD. The Vmax and Km of each reaction are shown at the bottom.