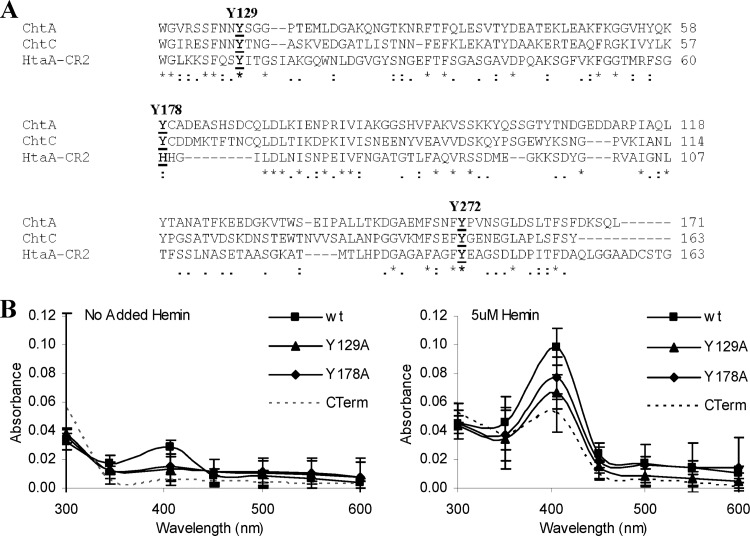
Fig 7.
(A) Sequence alignment of CR domains from the C. diphtheriae ChtA, ChtC, and HtaA proteins. Conserved tyrosine residues are indicated in bold and above the sequences. Asterisks indicate identity; colons and periods indicate sequence similarity. (B) Conserved tyrosine residues in the CR domain of ChtA are critical for optimal hemin binding. UV-visible spectroscopy was used to assess the hemin binding capability of proteins with various tyrosine-to-alanine substitutions in the CR domain of Strep-tagged ChtA. Proteins at 2 μM were incubated for 15 min in the presence of 5 μM hemin or with no added hemin prior to analysis. Values are the means from three independent experiments (± SD). The difference in peak absorbance (406 nm) between the wt and the Y129A mutant is significant at a P value of <0.01, and the difference in peak absorbance between wt and the Y178A, Y178H, and Y272A mutants is significant at a P value of <0.05.