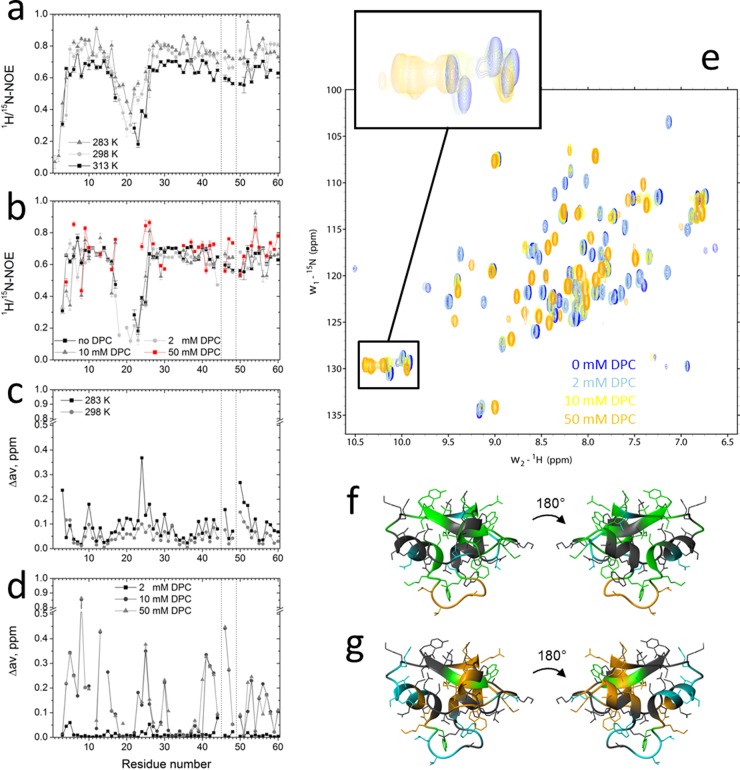
Fig 5.
15N relaxation and chemical-shift measurements of HM1 in solution and in the presence of DPC micelles. (a and b) 15N-heteronuclear NOE experiments with 0.3 mM uniformly 15N-labeled HM1 in solution at various temperatures (a) and in the presence of various DPC-d38 micelle concentrations at 313 K (b). (c and d) Normalized chemical shift perturbations (Δav) were calculated for 0.3 mM uniformly 15N-labeled HM1 in solution at different temperatures (c) and in the presence of various DPC-d38 micelle concentrations derived from 2D 1H-15N HSQC spectra and referenced to HM1 in solution at 313 K (d). The vertical dotted lines indicate proline residue locations. (e) 2D 1H-15N HSQC spectra of HM1 alone (blue) or in the presence of various concentrations of DPC-d38. The inset shows the chemical-shift perturbation of indole rings of tryptophan side chains of HM1 upon lipid interaction. (f) Ribbon representations of HM1 (PDB 2K35) summarizing amides and corresponding side chains of amino acid residues that exert significantly increased (green) or decreased (blue) 15N heteronuclear NOE values upon interaction with 50 mM DPC micelles. At 2 mM DPC concentration, the loop region from residues 18 to 25 (orange) revealed lipid binding participation. Residues that did not show significant changes are depicted in gray. (g) Ribbon representations of HM1 showing amides and corresponding side chains of amino acid residues that exert chemical perturbations of >0.1 ppm (green) or >0.2 ppm (orange) caused by interaction with 10 mM DPC micelles. Undetectable resonances caused by fast exchange between HM1 and 50 mM DPC micelles are colored blue. Residues that did not show significant changes are depicted in gray.