Abstract
A 1-year trial with entecavir plus adefovir resulted in a rate of virological response (VR) higher than that seen with lamivudine plus adefovir in multiple-drug-refractory chronic hepatitis B (CHB) patients. This extension study enrolled 89 of 90 patients who completed a 52-week randomized trial comparing treatment with entecavir plus adefovir (EA) to treatment with lamivudine plus adefovir (LA). At the baseline of the original study, all patients had lamivudine-resistant hepatitis B virus (HBV) and serum HBV DNA > 2,000 IU/ml despite prior lamivudine plus adefovir therapy. Of the 89 enrolled patients, 45 initially randomized to receive entecavir plus adefovir and the other 44 randomized to receive lamivudine plus adefovir received entecavir plus adefovir for an additional 52 weeks (EA-EA and LA-EA, respectively). The proportions of patients with a VR (serum HBV DNA < 60 IU/ml) gradually increased in both groups and were comparable at week 104 (42.2% in the EA-EA group and 34.1% in the LA-EA group; P = 0.51). The mean reductions in serum HBV DNA from baseline in the two groups were similar (−2.8 log10 IU/ml and −2.8 log10 IU/ml, respectively; P = 0.87). At week 104, the number of patients who retained the preexisting HBV mutants resistant to adefovir or entecavir had decreased from 8 to 2 in the EA-EA group and from 15 to 6 in the LA-EA group (P = 0.27). Both study groups had favorable safety profiles. In conclusion, up to 104 weeks of entecavir plus adefovir treatment was associated with a progressive VR, a decrease of levels of preexisting drug-resistant mutants, and no selection for additional resistance mutants of HBV in multiple-drug-refractory CHB patients. (This study has been registered at ClinicalTrials.gov under registration no. NCT01023217.)
INTRODUCTION
Approximately 400 million people worldwide are chronically infected with hepatitis B virus (HBV) (1, 2). The goal of HBV treatment is to prevent the development of cirrhosis, liver failure, and hepatocellular carcinoma (HCC). A high serum HBV DNA level in patients with chronic hepatitis B (CHB) is an independent risk factor for disease progression to cirrhosis and HCC (3, 4). Reducing HBV DNA to very low or undetectable levels with nucleos(t)ide analogue (NUC) therapy is associated with reduced risks of disease progression (5–8).
With the availability of potent NUCs, such as entecavir (ETV) and tenofovir disoproxil fumarate (TDF), suppression of serum HBV DNA to levels undetectable by PCR assays is achievable in most NUC treatment-naive patients without selecting for drug-resistant HBV mutants (9, 10). Until recently, however, many patients worldwide began antiviral treatment with the less potent NUCs, such as lamivudine (LAM) or adefovir (ADV), which also have a low genetic barrier to resistance.
Add-on combination therapy with LAM and ADV has been the most common rescue therapy in patients with LAM-resistant HBV. However, in such patients, the continued use of LAM does not provide additional antiviral suppression (11). Consequently, a substantial proportion of patients have a suboptimal virological response (VR) during LAM plus ADV combination therapy (12–14). The efficacy of ETV or TDF monotherapy may also be lower in patients who fail to respond to LAM plus ADV than in treatment-naive patients (15–17).
The combination of ETV, a nucleoside analogue, and ADV, a nucleotide analogue, has been shown to be a useful treatment option for patients who have failed various NUC therapies (18–20). This combination was assessed in a 52-week randomized trial in 90 CHB patients with suboptimal VRs after combination treatment with LAM plus ADV for lamivudine-resistant HBV (18). Patients treated with ETV plus ADV had a significantly higher rate of VR (serum HBV DNA < 60 IU/ml) than those who continued treatment with LAM plus ADV (29% versus 4%). At the end of the trial, additional mutations corresponding to resistance to ADV or ETV were detected in none of the patients given ETV plus ADV but were detected in 15% of patients given LAM plus ADV.
The purpose of this study was to evaluate the VR and resistance mutation profile during long-term extended treatment with ETV plus ADV in patients who completed the initial 52-week randomized trial.
MATERIALS AND METHODS
Study design.
This was an open-label trial in 90 LAM-resistant CHB patients who had failed to respond to LAM plus ADV combination therapy. The study cohort originally participated in a 52-week randomized controlled trial comparing the efficacy of ETV plus ADV with that of continued LAM plus ADV (18). All but one of the 90 subjects enrolled in this extension study and received ETV plus ADV for an additional 52 weeks (Fig. 1). All subjects who had participated in the initial study were eligible. There was no interruption in treatment before enrollment.
Fig 1.
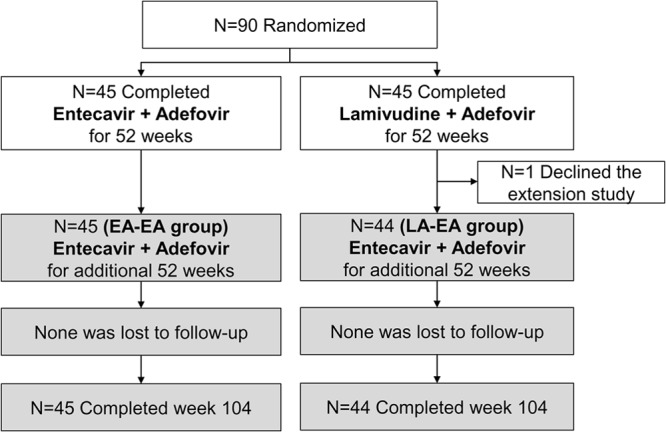
Flow diagram of the study. This extension study (gray boxes) enrolled 89 of 90 patients who completed the original 52-week randomized trial (white boxes) (18) in lamivudine-resistant chronic hepatitis B patients with a suboptimal response to lamivudine plus adefovir combination therapy.
This study (ClinicalTrials.gov identification [ID] number NCT01023217) was conducted in accordance with the ethical principles of the Declaration of Helsinki and in accordance with good clinical practice and local regulatory requirements. The Institutional Review Board of Asan Medical Center approved the study, and written informed consent was obtained from all patients.
Study subjects.
Patients eligible for the original study were aged between 16 and 75 years and were seropositive for hepatitis B surface antigen (HBsAg) for at least 6 months (18). They had confirmed HBV mutants resistant to LAM (rtM204V/I and/or rtL180M) and a serum HBV DNA concentration > 2,000 IU/ml after 6 months of combination treatment with LAM (100 mg/day) plus ADV (10 mg/day) that was ongoing at the time of randomization. All patients had good liver function (Child-Pugh-Turcotte score ≤ 6).
In the original study, 45 patients were randomized to receive treatment with ETV (1 mg/day orally) plus ADV (10 mg/day orally) for 52 weeks, and all agreed to extend the treatment to 104 weeks (EA-EA group; Fig. 1). A control group of 45 patients were initially treated with a continuation of LAM (100 mg/day orally) plus ADV (10 mg/day orally) for 52 weeks. All patients, except for one who refused to participate in the extension study, were switched to ETV (1 mg/day orally) plus ADV (10 mg/day orally) for an additional 52 weeks (LA-EA group).
Clinical and laboratory evaluations.
Patients were assessed on the first study visit (baseline) and at visits on weeks 4, 12, 24, 36, 52, 64, 76, 88, and 104. On each visit, patients were evaluated for adherence to study medications (checked by the returned pill count) and for adverse events. Hematology, biochemistry, and prothrombin time/international normalized ratio (INR) were assessed. The HBV DNA level was measured at baseline and at each study visit using a real-time PCR assay (Abbott Laboratories, Chicago, IL) with a linear dynamic detection range of 15 to 1 × 109 IU/ml. The HBV genome was assayed for ADV and ETV resistance mutations by restriction fragment mass polymorphism (RFMP) analysis at baseline and at weeks 52 and 104, as described previously (21, 22). HBeAg and anti-HBe were assessed at baseline and at weeks 52 and 104, using a commercially available enzyme immunoassay (Abbott Laboratories). The upper limit for normal alanine aminotransferase (ALT) was defined as 30 IU/liter for men and 19 IU/liter for women.
Statistical analysis.
The primary analysis set for determining efficacy and safety was a modified intent-to-treat population consisting of all patients who received at least one dose of study medication after enrollment. Patients who discontinued the study before week 104 were considered failures for all antiviral end points after the time of discontinuation.
Between-group differences of continuous variables were tested for significance using independent sample t tests. Categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate.
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC), SPSS version 13.0 (SPSS, Chicago, IL), and R version 2.13.2 (http://cran.r-project.org/). A P value of less than 0.05 was considered statistically significant.
RESULTS
Characteristics of patients.
The characteristics of the 90 patients who were randomized in the original study and the 89 who participated in the extension study are shown in Table 1. The two treatment groups were well-balanced for baseline characteristics, including HBeAg status and median serum HBV DNA concentration. However, after 52 weeks of treatment in the initial study, the residual median HBV DNA concentrations were significantly higher in the LA-EA group than in the EA-EA group (4.04 log10 IU/ml [interquartile range {IQR}, 3.35 to 4.93 log10 IU/ml] versus 2.60 log10 IU/ml [IQR, 1.59 to 3.27 log10 IU/ml]; P < 0.01).
Table 1.
Characteristics of study patientsa
| Characteristic | Value(s) |
|||||
|---|---|---|---|---|---|---|
| Baseline |
Wk 52 |
|||||
| LA-EA (n = 45) | EA-EA (n = 45) | P | LA-EA (n = 44) | EA-EA (n = 45) | P | |
| Age in yrs (mean ± SD) | 49 ± 11 | 45 ± 11 | 0.10 | 50 ± 11 | 46 ± 11 | 0.10 |
| Sex | ||||||
| No. (%) of males | 34 (75.6) | 33 (73.3) | 0.81 | 34 (77.3) | 33 (73.3) | 0.67 |
| No. (%) of females | 11 (24.4) | 12 (26.7) | 10 (22.7) | 12 (26.7) | ||
| Median (interquartile range) serum ALT (IU/liter) | 33 (25–47) | 28 (19–40) | 0.40 | 29 (21–41) | 23 (18–31) | 0.13 |
| Median (interquartile range) serum total bilirubin (mg/dl) | 1.0 (0.8–1.25) | 0.9 (0.7–1.2) | 0.07 | 1.2 (0.9–1.5) | 1.0 (0.9–1.3) | 0.10 |
| Median (interquartile range) serum albumin (g/dl) | 4.3 (4.2–4.5) | 4.3 (4.1–4.4) | 0.61 | 4.3 (4.1–4.5) | 4.3 (4.2–4.5) | 0.29 |
| Median (interquartile range) serum creatinine (mg/dl) | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.10 | 0.9 (0.8–1.1) | 0.9 (0.8–1.0) | 0.29 |
| Median (interquartile range) INR | 1.02 (0.98–1.07) | 1.01 (0.98–1.05) | 0.36 | 1.04 (1.00–1.08) | 1.04 (1.01–1.07) | 0.78 |
| No. (%) of patients with HBeAg positivity | 41 (91.1) | 39 (86.7) | 0.50 | 42 (96) | 40 (89) | 0.50 |
| Median (interquartile range) serum HBV DNA (log10 IU/ml) | 4.60 (3.93–5.25) | 4.40 (3.59–5.18) | 0.72 | 4.04 (3.35–4.93) | 2.60 (1.59–3.27) | <0.01 |
Baseline, week 0 in the original 52-week study (18). LA-EA, group of patients who were treated with combination of lamivudine (100 mg/day) and adefovir (10 mg/day) for 52 weeks and then with a combination of entecavir (1 mg/day) and adefovir for 52 weeks. EA-EA, group of patients who were treated with combination of entecavir (1 mg/day) and adefovir (10 mg/day) for 104 weeks. ALT, alanine aminotransferase; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; INR, international normalized ratio.
Virological response.
When the extension study began at week 52, significantly more patients in the EA-EA group than in the LA-EA group had achieved a VR (serum HBV DNA < 60 IU/ml, n = 13 [28.9%] versus n = 2 [4.4%]; P < 0.01) (Fig. 2). The number of patients in the EA-EA group who achieved a VR during treatment gradually increased to 19 (42.2%) by week 104 (Fig. 2). In the LA-EA group, the number of patients with a VR increased gradually after switching to treatment with ETV plus ADV at week 52. At week 104, the proportions of patients with VR were comparable in the EA-EA (42.2%) and LA-EA (n = 15; 34.1%) groups (P = 0.51) (Fig. 2).
Fig 2.
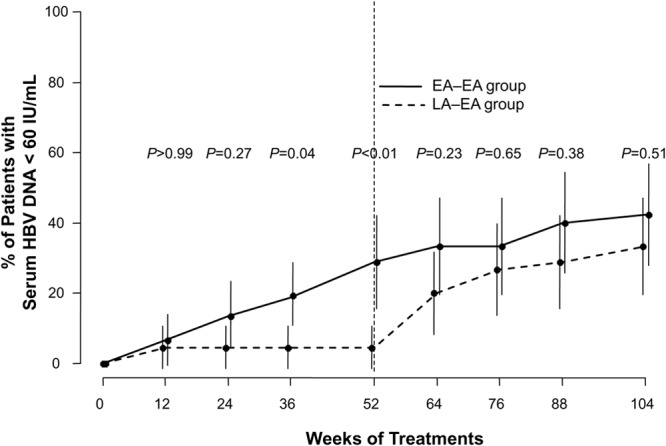
The proportions of patients with virological response (serum HBV DNA < 60 IU/ml) by study visit and treatment group. Baseline is defined as week 0 in the original 52-week study, and the data up to 52 weeks were adapted from the original study (18). The error bars indicate 95% confidence intervals. Fisher's exact test was used for the statistical analysis at each time point.
The mean reduction in serum HBV DNA levels from baseline was significantly less in the LA-EA group than in the EA-EA group at week 52 (−0.6 log10 IU/ml versus −2.2 log10 IU/ml; P < 0.01) (Fig. 3). However, the mean levels of HBV DNA reduction were similar in the two groups at week 64 and were −2.8 log10 IU/ml in the EA-EA group and −2.8 log10 IU/ml in the LA-EA group at week 104 (P = 0.87).
Fig 3.
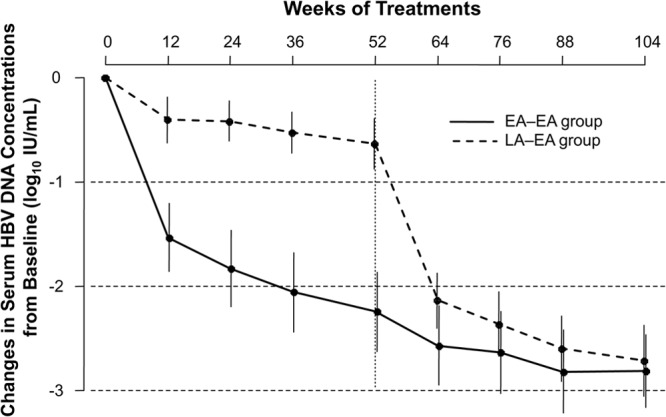
Mean change in serum HBV DNA concentrations from baseline over 104 weeks by study visit and treatment group. The error bars indicate 95% confidence intervals. An independent sample t test was used for the statistical analysis at each time point.
Virological breakthrough (≥1 log10 IU/ml increase in serum HBV DNA from nadir during therapy) was observed at week 88 in 1 patient in the EA-EA group who had HBV mutants resistant to ADV (rtA181T) in addition to mutants resistant to LAM (rtM204I and rtL180M) at baseline. Medication nonadherence was suspected in the patient at the time of virological breakthrough; no additional ADV- or ETV-resistant mutants were detected at that time or at the end of the study.
Biochemical and serological responses.
The proportions of patients with normal serum ALT concentrations at week 104 did not differ significantly in the LA-EA and EA-EA groups (59.1% versus 68.9%; P = 0.38). Among the patients who were HBeAg positive at baseline, 5.0% (2/40) in the LA-EA group and 15.4% (6/39) in the EA-EA group became HBeAg negative at week 104 (P = 0.15). The proportions of patients who achieved HBeAg seroconversion at week 104 were 5.0% (2/40) in the LA-EA group and 7.7% (3/39) in the EA-EA group (P = 0.68).
Genotypic resistance surveillance.
At baseline, all patients had LAM-resistant HBV mutants, including 64 (71.1%) with rtM204V/I plus rtL180M, 25 (27.8%) with rtM204V/I, and 1 (1.1%) with rtA181T plus rtL180M. Twenty patients also had ADV-resistant HBV mutants with or without ETV-resistant mutants, including 18 patients with ADV-resistant HBV mutants (10 with rtA181V/T plus rtN236T, 8 with rtA181V/T), 1 patient with an ETV-resistant mutant (rtT184A), and 1 patient with a mutant that was both ADV resistant and ETV resistant (rtA181T plus rtN236T plus rtM250L) (Table 2). The numbers of patients with baseline ADV-resistant and/or ETV-resistant HBV mutants in the LA-EA group (n = 12; 26.7%) and EA-EA groups (n = 8; 17.8%) were not significantly different (P = 0.31).
Table 2.
Genotypic resistance surveillancea
| Parameter | No. (%) of patients with indicated value(s)/total no. of patients |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Wk 52 |
Wk 104 |
|||||||
| LA-EA | EA-EA | P | LA-EA | EA-EA | P | LA-EA | EA-EA | P | |
| Patients with HBV mutants resistant to ADV or ETV | 12 (26.7) | 8 (17.8) | 0.31 | 15 (33.3) | 3 (6.7) | <0.01 | 6 (13.3) | 2 (4.4) | 0.27 |
| Patients with retainment of indicated baseline HBV mutant(s) | 12 | 8 | 0.31 | 10/12 (83.3) | 3/8 (37.5) | 0.06 | 6/12 (50) | 2/8 (25) | 0.37 |
| rtA181T,V + rtN236T | 3 | 0 | 3 | 0 | 3 | 0 | |||
| rtA181T + rtN236T | 3 | 1 | 3 | 0 | 1 | 0 | |||
| rtA181V + rtN236T | 0 | 3 | 0 | 1 | 0 | 1 | |||
| rtA181T | 4 | 1 | 2 | 0 | 0 | 0 | |||
| rtA181V | 2 | 1 | 2 | 0 | 2 | 0 | |||
| rtT184A | 0 | 1 | 0 | 1 | 0 | 0 | |||
| rtA181T + rtN236T + rtM250L | 0 | 1 | 0 | 1 [rtM250L] | 0 | 1 [rtM250L] | |||
| Patients with additional emergence of indicated HBV mutant(s) | NA | NA | NA | 5/33 (15.2) | 0/38 (0) | 0.02 | 0/33 | 0/38 | NA |
| rtA181T + rtN236T | NA | NA | 1 | 0 | 0 | 0 | |||
| rtA181V + rtN236T | NA | NA | 2 | 0 | 0 | 0 | |||
| rtA181T | NA | NA | 1 | 0 | 0 | 0 | |||
| rtN236T | NA | NA | 1 | 0 | 0 | 0 | |||
Baseline, week 0 in the original 52-week study (18). NA, not applicable.
The ADV and ETV resistance mutations in paired samples from all study patients with detectable serum HBV DNA at baseline, week 52, and week 104 were genotyped by RFMP (Table 2). At week 52, 83.3% (10/12) of the patients in the LA-EA group and 37.5% (3/8) of those in the EA-EA group with ADV- or ETV-resistant HBV mutants at baseline still had them (P = 0.06). ADV resistance mutations were additionally selected in 5 patients in the LA-EA group and in none in the EA-EA group at week 52 (P = 0.02). Four of the five patients also had inadequate VRs at that time. The overall number of patients with detectable ADV or ETV resistance mutations at week 52 was significantly greater in the LA-EA group than in the EA-EA group (n = 15 [33.3%] versus 3 [6.7%]; P < 0.01) (Table 2 and Fig. 4).
Fig 4.
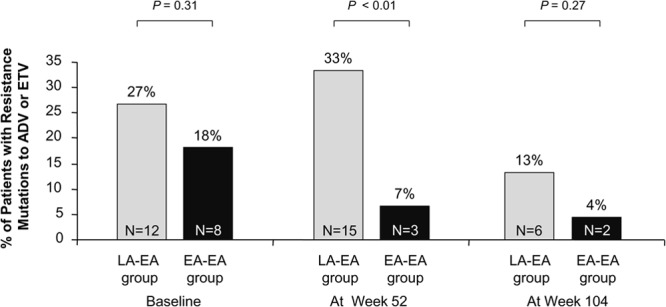
The proportions of patients with detectable HBV mutants resistant to adefovir or entecavir by study visit and treatment group. Fisher's exact test was used for the statistical analysis at each time point.
Between weeks 52 and 104, no additional ADV and/or ETV resistance mutation was selected in any patient in either group. Only 8 of the 20 patients with ADV or ETV resistance mutations at baseline still had them at week 104 (P < 0.01). Thus, the overall numbers of patients with detectable ADV or ETV resistance mutations at week 104 were significantly lower than those measured at baseline (P < 0.01) and were comparable in the two groups (6 in the LA-EA group and 2 in the EA-EA group; P = 0.27) (Table 2 and Fig. 4).
Safety.
The numbers of patients with any adverse event were similar in the two groups (n = 16 [36.4%] versus 15 [33.3%]; P = 0.76) (Table 3). Most adverse events were mild (grade 1) and considered unrelated to treatment. No patient experienced an ALT flare (>10× the upper limit of normal [ULN]) or a decrease of the serum phosphorus level to <2.0 mg/dl during the treatment period. One patient in the LA-EA group and three patients in the EA-EA group required a reduction of the ADV dose following an increase in the serum creatinine concentration. The dose adjustment led to a return to normal creatinine levels in all four patients. The numbers of patients with serious adverse events (SAE) were also similar in the two groups (n = 4 [9.1%] versus 5 [11.1%]; P > 0.99); all SAE were considered unrelated to treatment. Two patients in the EA-EA group developed HCC.
Table 3.
Summary of safety
| Parameter | Value(s) at wk 104 |
||
|---|---|---|---|
| LA-EA (n = 44) | EA-EA (n = 45) | P | |
| No. (%) of patients with: | |||
| Any adverse event | 16 (36.4) | 15 (33.3) | 0.76 |
| Serious adverse event | 4 (9.1) | 5 (11.1) | 1.00 |
| Hepatocellular carcinoma | 0 | 2 (4.4) | 0.49 |
| Dose reduction of study medication | 1 (2.3) | 3 (6.7) | 0.62 |
| Discontinuation of study medication | 0 | 0 | NA |
| ALT flarea | 0 | 0 | NA |
| Increase in serum creatinineb | 1 (2.3) | 2 (4.4) | 1.00 |
| Mean ± SD serum creatinine (mg/dl) | 0.96 ± 0.21 | 0.91 ± 0.23 | 0.26 |
| Mean ± SD serum phosphorus (mg/dl) | 3.32 ± 0.46 | 3.34 ± 0.48 | 0.86 |
| Mean ± SD serum lactic acid (mmol/liter) | 1.50 ± 0.82 | 1.43 ± 0.74 | 0.70 |
ALT flare, ALT > 10 × ULN.
Increase in serum creatine, ≥0.5 mg/dl above baseline.
DISCUSSION
In the initial phase of this study, 52 weeks of treatment with ETV plus ADV resulted in a higher VR rate than was achieved with LAM plus ADV and in significantly suppressed HBV replication and was not associated with additional selection of resistance mutations (18). However, ETV plus ADV combination therapy was not sufficiently effective to achieve VR in all patients. The patients' histories of multiple-drug refractoriness and the relatively short duration of the treatment were suggested to have reduced the treatment effectiveness. Thus, in this extension study, we wanted to observe the efficacy of long-term ETV plus ADV combination treatment. The study showed that prolonged treatment with the combination of ETV plus ADV for up to 104 weeks resulted in a gradual increase of the VR rates in the patients. Extensive genotypic analyses confirmed that no additional selection of mutations corresponding to resistance to ADV or ETV occurred during treatment.
In the initial phase of the study, up to 78% of the patients who continued on LAM plus ADV had inadequate VRs at week 52 (18). However, after switching to treatment with ETV plus ADV, those patients achieved rapid reductions of serum HBV DNA load and significantly increased VR rates. Together with those of another large-scale randomized trial comparing the efficacy of ETV plus ADV to the efficacy of LAM plus ADV in patients with LAM resistance (20), these data clearly demonstrate the superiority of ETV plus ADV combination therapy in suppressing HBV replication in CHB patients with drug-resistant HBV mutants. These data also confirm the incremental benefit of combination therapy with ETV plus ADV, two agents that have independent antiviral potency without cross-resistance.
Patients in the LA-EA group achieved rapid reductions in the serum HBV DNA load immediately after switching to treatment with ETV plus ADV after 52 weeks of treatment with LAM plus ADV. Nevertheless, late implementation of this rescue therapy should not be advocated, because continuing treatment with LAM plus ADV after a suboptimal response during the initial phase of this study promoted the selection of multiple-drug-resistant HBV strains. In those patients, continuing treatment with LAM plus ADV selected for mutations resistant to ADV in 15% (5/33) of the patients by week 52. In general, the efficacy of any rescue therapy decreases as the number of genotypic resistance mutations increases (17, 19). Rescue therapy should thus be implemented as soon as possible in patients with multiple-drug failure, before the selection of mutations corresponding to resistance to multiple drugs.
Genotypic analysis of HBV drug resistance mutations in all patients with detectable serum HBV DNA at week 104 did not demonstrate selection of additional ADV or ETV resistance mutations. The analysis revealed that a significant reduction of levels of detectable ADV- and/or ETV-resistant mutants had occurred. One patient who had ADV-resistant HBV mutant at baseline experienced virological breakthrough during treatment with ETV plus ADV. The response in the patient was thought to be associated with medication nonadherence and was not related to selection of additional resistance mutations. These results may be associated with the lack of cross-resistance between ETV and ADV and the susceptibility of ADV-resistant HBV mutants to ETV, as shown by both laboratory and clinical studies (15, 23, 24). The efficacy of ETV plus ADV combination therapy in preventing additional selection of resistance mutations was also demonstrated in a recent large-scale randomized trial in patients with LAM resistance (20). In this trial, genotypic resistance to ETV or ADV was detected in only 2 of 138 patients after treatment with ETV plus ADV for 96 weeks. One of the two patients already harbored an ADV-resistant mutant at baseline.
The combination of ETV and ADV was generally well tolerated during the 104-week treatment period. A few patients (one patient in the LA-EA group and three patients in the EA-EA group) required a dose reduction of ADV after a mild increase in the level of serum creatinine. Recovery of normal creatinine levels occurred in all four patients. Overall, there were no significant changes in serum creatinine or serum phosphorus concentrations.
Treatment options for patients with multiple-drug-refractory or -resistant HBV are limited, and even the few options that exist have undergone limited evaluation in randomized, controlled clinical trials. Although recent studies suggest that TDF may be effective for the treatment of patients with LAM resistance (25), several recent single-arm cohort studies demonstrated that the efficacy of TDF monotherapy was reduced by a history of suboptimal response to multiple drugs and by the presence of ADV resistance mutations (16, 17). Furthermore, TDF is still not available in some countries, and to some patients, because of licensing, cost, or tolerability. This study may have inherent limitations because it was an open-label evaluation. Although objective virological and biochemical end points were used and drug adherence was monitored, the lack of blinding might have affected the attitudes of the study patients or investigators.
In conclusion, this extension study demonstrated that 104 weeks of treatment with ETV plus ADV resulted in a gradual increase of the VR rate, a steady reduction in the serum HBV DNA concentration, a reduction in levels of preexisting antiviral drug-resistant HBV mutants, and no occurrence of additional drug resistance mutations after the initial 52 weeks of treatments. The safety profile was acceptable. These results support the combination of ETV plus ADV as a long-term treatment option for CHB patients with multiple-drug resistance or refractoriness.
ACKNOWLEDGMENTS
This work was supported by Bristol-Myers Squibb, which also provided the study drugs (lamivudine, adefovir, and entecavir). Bristol-Myers Squibb was permitted to review the manuscript and suggest changes, but had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The final decision on content was exclusively retained by us.
We thank Seungbong Han for his excellent help in statistical analyses.
Y.-S. Lim was responsible for the concept and design of the study, the acquisition, analysis, and interpretation of the data, and the drafting of the manuscript. J.Y. Lee was involved in the acquisition of the data and administrative and technical support. D. Lee, J.H. Shim, H.C. Lee, and Y.-S. Lee helped with the acquisition of the data and critical revision of the manuscript for important intellectual content. D.J. Suh supervised the study and provided critical revision of the manuscript.
Y.-S. Lim has received grant support from Bristol-Myers Squibb and Gilead Sciences. The remaining authors of this article declare that we have no conflicts of interest.
Footnotes
Published ahead of print 6 May 2013
REFERENCES
- 1. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. 2006. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J. Hepatol. 45:529–538 [DOI] [PubMed] [Google Scholar]
- 2. Schiff ER. 2006. Prevention of mortality from hepatitis B and hepatitis C. Lancet 368:896–897 [DOI] [PubMed] [Google Scholar]
- 3. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. 2006. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295:65–73 [DOI] [PubMed] [Google Scholar]
- 4. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. 2006. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130:678–686 [DOI] [PubMed] [Google Scholar]
- 5. Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. 2010. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52:886–893 [DOI] [PubMed] [Google Scholar]
- 6. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. 2004. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 351:1521–1531 [DOI] [PubMed] [Google Scholar]
- 7. Toy M, Veldhuijzen IK, de Man RA, Richardus JH, Schalm SW. 2009. Potential impact of long-term nucleoside therapy on the mortality and morbidity of active chronic hepatitis B. Hepatology 50:743–751 [DOI] [PubMed] [Google Scholar]
- 8. Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio PL, Rizzetto M, Craxi A. 2004. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 40:883–891 [DOI] [PubMed] [Google Scholar]
- 9. Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, Zhang H, Tenney DJ, Tamez R, Iloeje U. 2010. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 51:422–430 [DOI] [PubMed] [Google Scholar]
- 10. Snow-Lampart A, Chappell B, Curtis M, Zhu Y, Myrick F, Schawalder J, Kitrinos K, Svarovskaia ES, Miller MD, Sorbel J, Heathcote J, Marcellin P, Borroto-Esoda K. 2011. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology 53:763–773 [DOI] [PubMed] [Google Scholar]
- 11. Peters MG, Hann HW, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray DF, Sullivan M, Kleber K, Ebrahimi R, Xiong S, Brosgart CL. 2004. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126:91–101 [DOI] [PubMed] [Google Scholar]
- 12. Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. 2007. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology 45:307–313 [DOI] [PubMed] [Google Scholar]
- 13. Lampertico P, Vigano M, Manenti E, Iavarone M, Sablon E, Colombo M. 2007. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology 133:1445–1451 [DOI] [PubMed] [Google Scholar]
- 14. Heo NY, Lim YS, Lee HC, Chung YH, Lee YS, Suh DJ. 2010. Lamivudine plus adefovir or entecavir for patients with chronic hepatitis B resistant to lamivudine and adefovir. J. Hepatol. 53:449–454 [DOI] [PubMed] [Google Scholar]
- 15. Shim JH, Suh DJ, Kim KM, Lim YS, Lee HC, Chung YH, Lee YS. 2009. Efficacy of entecavir in patients with chronic hepatitis B resistant to both lamivudine and adefovir or to lamivudine alone. Hepatology 50:1064–1071 [DOI] [PubMed] [Google Scholar]
- 16. Patterson SJ, George J, Strasser SI, Lee AU, Sievert W, Nicoll AJ, Desmond PV, Roberts SK, Locarnini S, Bowden S, Angus PW. 2011. Tenofovir disoproxil fumarate rescue therapy following failure of both lamivudine and adefovir dipivoxil in chronic hepatitis B. Gut 60:247–254 [DOI] [PubMed] [Google Scholar]
- 17. van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Huppe D, Stein K, Trojan J, Sarrazin C, Bocher WO, Spengler U, Wasmuth HE, Reinders JG, Moller B, Rhode P, Feucht HH, Wiedenmann B, Berg T. 2010. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 51:73–80 [DOI] [PubMed] [Google Scholar]
- 18. Lim YS, Lee JY, Lee D, Shim JH, Lee HC, Lee YS, Suh DJ. 2012. Randomized trial of entecavir plus adefovir in patients with lamivudine-resistant chronic hepatitis B who show suboptimal response to lamivudine plus adefovir. Antimicrob. Agents Chemother. 56:2941–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim YS, Lee TH, Heo NY, Shim JH, Lee HC, Suh DJ. 2012. Entecavir plus adefovir combination treatment for chronic hepatitis B patients after failure of nucleoside/nucleotide analogues. Antivir. Ther. 17:53–60 [DOI] [PubMed] [Google Scholar]
- 20. Heo J, Ahn SH, Kweon YO, Kim BH, Chan HL-Y, Horban A, Wongcharatrawee S, Llamoso C, Lee KS. 2012. Entecavir+adefovir versus lamivudine+adefovir or entecavir alone in lamivudine-resistant chronic hepatitis B: 96-week data from the DEFINE study. Hepatology 56:392A. [DOI] [PubMed] [Google Scholar]
- 21. Han KH, Hong SP, Choi SH, Shin SK, Cho SW, Ahn SH, Hahn JS, Kim SO. 2011. Comparison of multiplex restriction fragment mass polymorphism and sequencing analyses for detecting entecavir resistance in chronic hepatitis B. Antivir. Ther. 16:77–87 [DOI] [PubMed] [Google Scholar]
- 22. Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. 2006. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 43:1385–1391 [DOI] [PubMed] [Google Scholar]
- 23. Reijnders JG, Deterding K, Petersen J, Zoulim F, Santantonio T, Buti M, van Bommel F, Hansen BE, Wedemeyer H, Janssen HL. 2010. Antiviral effect of entecavir in chronic hepatitis B: influence of prior exposure to nucleos(t)ide analogues. J. Hepatol 52:493–500 [DOI] [PubMed] [Google Scholar]
- 24. Qi X, Xiong S, Yang H, Miller M, Delaney WE, IV 2007. In vitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir. Ther. 12:355–362 [PubMed] [Google Scholar]
- 25. Fung S, Kwan P, Fabri MJ, Horban A, Pelemis M, Husa P, Hann HW, Flaherty JF, Massetto B, Dinh P, Corsa A, Kitrinos K, McHutchison JG, Gane E. 2012. Efficacy and safety of tenofovir DF (TDF) in chronic hepatitis B virus infected patients with documented lamivudine resistance. Hepatology 56:200A [Google Scholar]


